Drug Safety and Pharmacovigilance: Scope, Types, Careers, and Certification Guide
Just a few years ago, when the world witnessed the global pandemic, COVID-19, millions of people were losing their lives. That was when the vaccines were released. It brought a sense of hope, along with a bit of fear. Behind every approved shot was a group of unsung heroes who worked around the clock. When the reports of rare side effects emerged, it was drug safety and pharmacovigilance that helped regulators identify patterns, assess risks, and update safety guidelines within days.
Today, every pill, injection, and therapy that we take has to pass through an intricate safety web where data is saving lives. This global vigilance network stretches from WHO’s Uppsala Monitoring Centre in Sweden to regional drug safety teams in India and the US.
Welcome to the world of Drug Safety and Pharmacovigilance, a science that never sleeps. With the increasing use of medicine worldwide, the demand for skilled professionals who can monitor and report drug safety issues is growing rapidly.
Let us dive into this world deeper and explore the scope, types, careers, and certifications in this fast-growing industry. Here is your opportunity to be part of modern healthcare safety.
Scope of Pharmacovigilance: More Than Just Monitoring Side Effects
Do you know that Pharmacovigilance is not just about recording side effects of the drugs? It’s far beyond. From clinical trials to post-market surveillance, it covers every stage of the drug process.
To help you understand better, take a look at the list where Pharmacovigilance plays a role:
- Adverse Drug Reactions (ADRs) and drug interactions
- Medication errors, counterfeit drugs, and product quality defects
- Misuse, abuse, or lack of efficacy
- Vaccine and biological product monitoring
Today, Pharmacovigilance has a wide reach in healthcare. From prescription medicines, vaccines, over-the-counter medications, to medical devices, and even herbal medicines, Pharmacovigilance is touching every corner.
Were you aware? WHO Programme for International Drug Monitoring and the Uppsala Monitoring Center in Sweden coordinate data from over 150 countries with the world’s largest adverse drug reaction database, known as VigiBase. And to strengthen this system further, national regulatory agencies such as the FDA, EMA, and MHRA are enforcing strict Good Pharmacovigilance Practices (GVPs).
With this, you can understand the expanding global scope for pharmacovigilance. This is not just a scientific field but a public health mission that ensures safer healthcare for billions across the world.
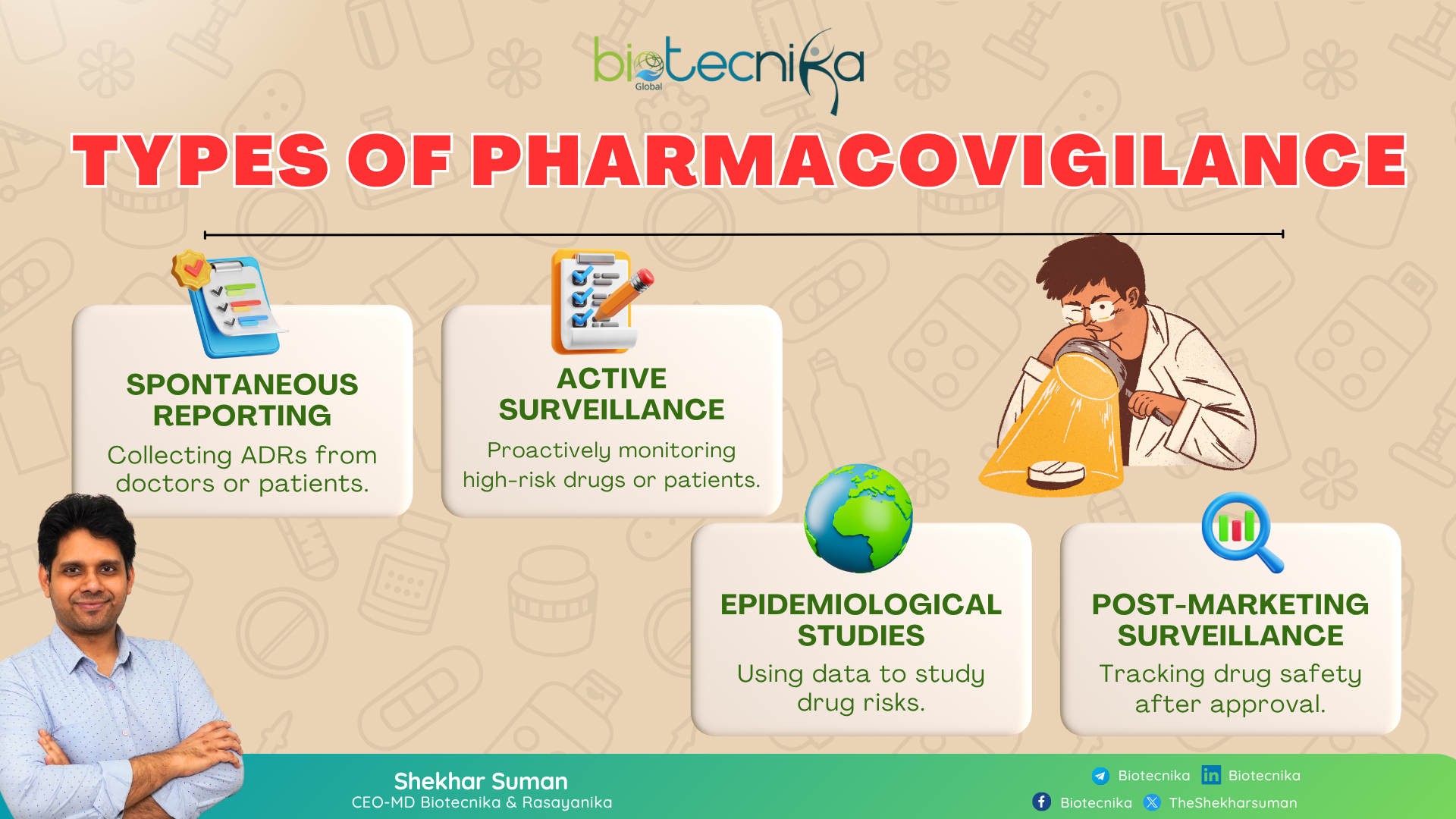
Types of Pharmacovigilance: How Safety Data is Collected and Analyzed
The pharmacovigilance experts use several different strategies and study designs to make sure every drug that reaches the public is safe. Let’s break down the types of pharmacovigilance in a simple way:
-
Spontaneous Reporting (Passive Surveillance)
Every time you report adverse drug reactions (ADRs) to regulatory agencies or pharmaceutical companies, these experts track the drug safety. This is a classic method.
Example: When a doctor or patient reports an unexpected reaction from a newly approved drug to the national safety database.
-
Active or Targeted Surveillance
Instead of waiting for reports, in this approach, the experts proactively track specific groups of patients or high-risk medicines through organized programs like Cohort Event Monitoring (CEM). You can observe this approach in the case of vaccines, oncology drugs, or gene therapies, where close monitoring is important.
-
Epidemiological Studies
These include case-control, cohort, or population-based studies that use large healthcare databases to identify and quantify risk factors or adverse outcomes.
-
Post-Marketing Surveillance (PMS)
Once the drug reaches the market, it’s continually monitored through Periodic Safety Update Reports (PSURs) and Risk Management Plans (RMPs). This collection of reports helps detect long-term or rare safety issues that might not appear during clinical trials.
Every pharmacovigilance method depends on one common factor: high-quality, accurate data. Even a single misreported case and lead to whole safety analyses. That’s where regulatory authorities come into the picture. With their strong training and data validation processes, they ensure the safety of drugs.
Careers in Drug Safety and Pharmacovigilance: Global Demand, Real Impact
The global PV market is projected to grow to USD 7.9 billion in 2024. The experts have estimated that the market will expand to over USD 11.7 billion by 2030. This growth highlights the rising regulatory scrutiny, new drug launches, and an increasing emphasis on patient safety.
If you are a life science graduate looking for a promising career that combines science, data, and public health, then here is a field that offers you tremendous opportunities.
Here’s a list of jobs in the field of drug safety and pharmacovigilance, and what they are involved in:
| Role/Title | Primary Responsibilities |
| Drug Safety Associate / Officer | Process individual adverse event reports, perform MedDRA coding, and manage data entry into PV systems like Oracle Argus or ArisGlobal. |
| Pharmacovigilance Specialist / Scientist | Conduct signal detection, aggregate analysis, prepare safety reports (PSURs/PBRERs), and collaborate with clinical and regulatory teams. |
| Pharmacovigilance Manager | Oversee PV operations, supervise safety teams, manage vendor relationships, and ensure inspection readiness. |
| Global Safety Lead / QA Manager | Direct international safety surveillance, implement risk minimization strategies, and maintain global compliance. |
| EU QPPV (Qualified Person for Pharmacovigilance) | Hold legal responsibility for the company’s pharmacovigilance system in the EU, ensuring 24/7 regulatory oversight. |
| Medical Director / PV Director | Define safety strategies, liaise with regulatory authorities, and integrate safety insights into drug development programs. |
Educational background:
Most of the PV professionals come from different fields such as life science, pharmacy, and medicine. Their core skills include data analysis, regulatory knowledge (ICH, FDA, EMA), and familiarity with pharmacovigilance databases.
The field also attracts clinical researchers and bioinformaticians, as AI and machine learning tools are now used in pattern recognition. With AI and ML, the process is now faster than ever before.
In short, a career in pharmacovigilance lets you protect lives while advancing global healthcare.
Drug Safety Pharmacovigilance Certification: Boosting Your Global Career
If you are serious about entering or advancing in this field, pursuing a drug safety pharmacovigilance certification can give you a strong edge. These certifications not only validate your technical knowledge but also improve your employability in multinational pharmaceutical and regulatory settings.
| Program / Certification | Provider / Institution | Key Highlights |
| Graduate Certificate in Drug Safety & Pharmacovigilance | University of Georgia (USA) | 17-credit online program covering global regulatory frameworks, safety data analysis, and reporting. |
| Safety and Pharmacovigilance Certificate | Drug Information Association (DIA) | Competency-based program offering up to 64 Continuing Education credits on PV regulations and best practices. |
| Advanced PV & Regulatory Affairs Certification | Center for Clinical Research & Professional Studies (CCRPS) | Accredited online certification with 169 self-paced modules and CME credits. |
| Pharmacovigilance Hands-on Training Program with Project Work + 100% Placement Assistance | Biotecnika | Focused on ICSR reporting, signal detection, and PV data management for beginners. |
As a young graduate, these programs will help you understand the global safety requirements and transition smoothly into regulatory or corporate PV teams.
With time, the regulatory guidelines and WHO PV manuals are evolving continuously, and these programs will help you stay up to date with them. To gain more knowledge in this field, you can attend international conferences. Along with them, even scientific journals will help you stay ahead in this fast-changing field.
Why Pharmacovigilance Matters More Than Ever?
Pharmacovigilance is not just a compliance requirement. Today, it’s a global safety network that is protecting millions of lives across the globe. From personalized medicine to AI-driven drug development, every day, many new therapies are emerging. PV professionals make sure all these innovations are safe and support the patient’s well-being.
And data accuracy is the heart of this global mission. A well-trained PV professional knows that every report, every coded event, and every follow-up matters. It’s not just paperwork. It’s science in action.
For life science and graduates, pharmacovigilance offers a future-proof, meaningful, and globally respected career path.
In Conclusion
As we know now, drug safety and pharmacovigilance are the silent safety systems of global healthcare. The scope of pharmacovigilance talks about every drug, every region, and every patient. The types of pharmacovigilance activities ensure that no safety signal goes unnoticed. And for aspiring young minds, a variety of drug safety and pharmacovigilance jobs and certifications offer a direct route into this impactful field.
Whether you are aiming to be a Drug Safety Associate or a Global PV Director, every report you review, every safety signal you validate, and every risk you mitigate helps make medicine safer for mankind.
In a world of innovations, pharmacovigilance is the conscience of medicine, where science meets responsibility.