Over the past few years, there has been a significant growth in clinical research, as it was noticed that the number of clinical trials registered worldwide has increased in the last decade by more than 50%. It is because of the rising demand for personalized medicines, drugs, new therapies, and global trials. This suggests that clinical research careers are going to dominate and will be a highly demanding career in the future at the global level.
According to Zion Market Research, the clinical trials market globally was valued at more than 50 billion Dollars in 2022 and is expected to increase to more than 90 billion Dollars by 2030. The rise in demand for clinical research will create abundant opportunities for clinical research careers globally.
What is Clinical Research?
Clinical research is one of the vital parts of the Biomedical research subject. This involves testing of newly discovered drugs, therapies, and devices on subjects like animal models and humans, to ensure the safety and efficacy of the product before introducing it to a larger population.
In this article, we will be discussing the clinical research careers from entry-level jobs, Clinical Research Associate (CRA), to advanced-level jobs, Project Manager or Director Positions.
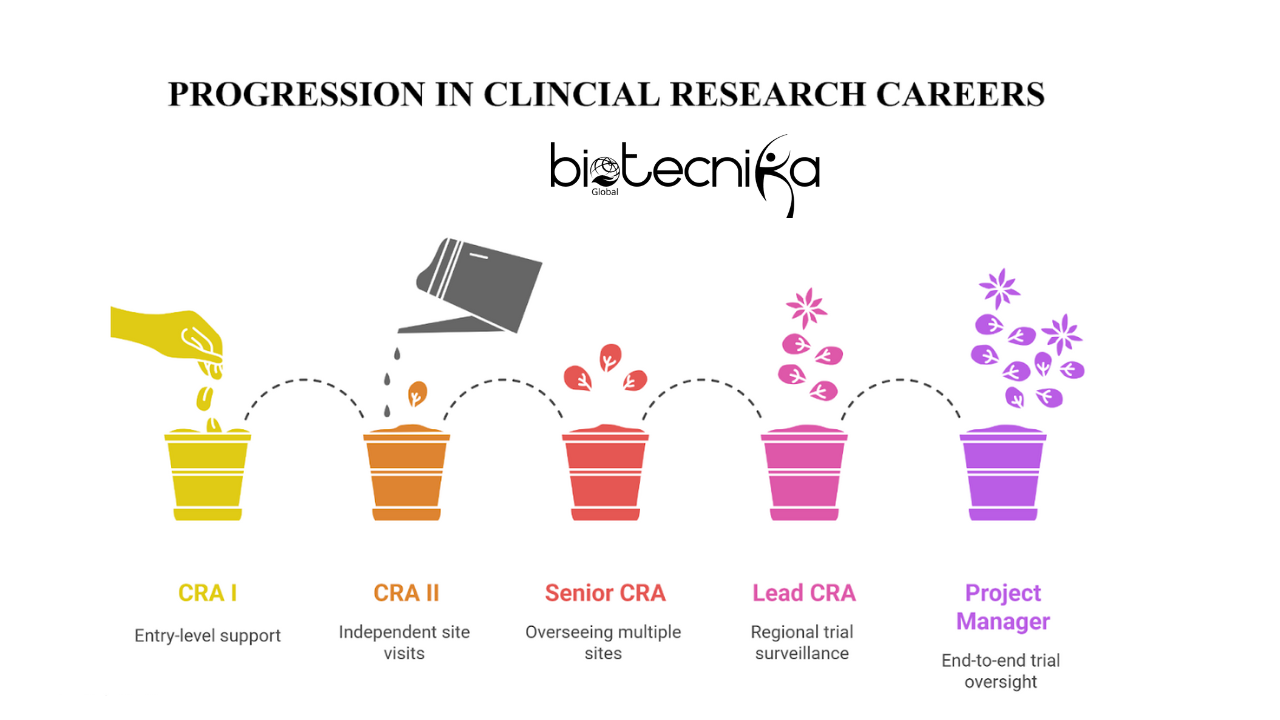
There are five levels of Clinical research careers: CRA I, CRA II, Senior CRA, Lead CRA, and Clinical Project Manager.
Progression of Clinical Research
Lead CRA → Project Manager → Clinical Operations Director
1) CRA I
This is an entry-level position in clinical research careers, also referred to as CRA I. They support clinical trial activities, assist in document maintenance, monitor sites for compliance, and coordinate patient visits.
Clinical Research Position Role & Responsibilities
- They are responsible for site initiation, monitoring routinely under the supervision of a senior CRA.
- They ensure compliance through clinical trials with protocols and Good Clinical Practice ( GLP) by monitoring the sites routinely.
- They review the trial documents and ensure that they collect accurate data from source data against the Case Report Form (CRFs).
- They report their collected data from the trials to the senior CRA or Project Manager.
- They help seniors to resolve site issues and in communication between the sponsor ( Biotech/ Pharma companies) and the Study sites ( Hospitals/ Private labs).
Qualifications
- Bachelor’s in Life Sciences, Pharmacy
- 0-2 years of experience
- Previous clinical research experience will be beneficial
Skills
- ICH- GCP regulatory guidelines
- Clinical Research Phases, Trial Systems
- Analytical, problem-solving, and decision-making skills
2) CRA II
This is a senior-level position in clinical research careers after CRA I. They work independently on the sites, e.g, monitoring, site initiation, and close-out visits.
Clinical Research Position Role & Responsibilities
- Independently visit the trial sites routinely to ensure the process follows regulatory guidelines and is performed ethically.
- Ensuring the trial data of the patient is accurate.
- Collaboration with stakeholders and acting as a medium between the site coordinator and the sponsors
- Guide and mentor the CRA I and report the collected data to senior management.
- They review and maintain the trial documents and escalate the complex issues to the seniors.
Qualifications
- Bachelor’s Degree in Life Science, Pharmacy
- 2-4 years of experience as CRA I level or equivalent
Skills
- ICH-GCP regulatory guidelines
- Clinical research phases and systems
- Analytical, problem-solving, and decision-making skills
- Ability to travel frequently
3) Senior CRA
The CRA that have more than 5 years of experience with leadership and mentorship qualities is eligible for this senior-level position. They work independently and handle more than one clinical trial site.
Clinical Research Position Role & Responsibilities
- Unlike entry-level CRAs, they are responsible for site initiation, but site selection and patient recruitment for the trial.
- They will independently oversee multiple sites and handle complex trial sites.
- Risk management and mentorship to entry-level and mid-level CRAs
- Escalating issues to higher management and solving site issues.
- Site performance reviewing and monitoring the site regularly to ensure compliance
- Prepare monitoring reports and make sure that the data of patients is accurate by cross-checking against the CRFs.
Qualifications
- Bachelor’s degree in Life Science, Pharmacy
- Master’s degree (Preferred) in Life Science, Pharmacy
- More than 5 years of experience as CRA I / CRA II
Skills
- ICH-GCP regulatory guidelines
- Clinical research phases, trial system
- Advanced regulatory knowledge
- Mentorship quality
4) Lead CRA
This is an experienced-level position of CRA responsible for the surveillance of the trial process, from beginning to end.
Clinical Research Position Role & Responsibilities
- They are responsible for site selection, initiation, and the patient recruitment process.
- They will review the monitoring reports and ensure that the trials are followed under compliance within a region.
- They act as the communicator between the trial site, CRA, and the manager.
- They will manage multiple sites within a region and resolve site challenges.
- Mentoring or guidance to Junior CRA
Qualifications
- Bachelor’s degree in Life Science, Pharmacy, Nursing
- Master’s degree (Preferred) in Life Science, Pharmacy, Nursing
- 5-7 years of experience as CRA I / CRA II / Senior CRA
Skills
- ICH-GCP Guidelines
- Advanced regulatory protocols
- Ability to manage a complex site
- Leadership and communication
5) Clinical Project Manager
This is a senior position after CRA in the hierarchy of clinical research careers, but the role at the top is that of a director of clinical operations.
Clinical Research Position Role & Responsibilities
- They are responsible for the entire trial process from beginning to end, unlike the CRA, who is responsible for site monitoring.
- They will ensure that the project is completed within a decided timeframe and budget.
- They ensure the trials follow compliance and ethics.
- They will report the trial progress to sponsors.
- They will interact among CRAs, Medical writers, and data management teams (cross-functional team).
Qualifications
- BSc / MSc (preferred)/ PhD/ MBA in Life Science, Pharmacy, Nursing, or related fields.
- 8-12 years of experience as CRA I / CRA II / Senior CRA/ Trial Manager
Skills
- ICH-GCP guidelines and advanced regulatory guidelines.
- Leadership quality and management
- Risk assessment and communication
A clinical trial is the backbone of clinical research, which is one of the pillars of growth of the healthcare industry. It leads to innovations and contributes to scientific knowledge through discoveries or by renovating the already existing ones. The increasing demand for clinical trials worldwide will open the door for clinical research careers. In this article, we have discussed the clinical research careers from CRA to the Project Manager position.
The CRA will be responsible for site monitoring and resolving only the site issues, but a Manager will oversee and manage the entire project. Each of the roles is vital and is necessary for the success of the clinical research process. Whether you choose to start your journey from CRA to aim to become the director of operations in the clinical research field, the passage will create career advancement and contribute to the medical science community.