CRO Career In Biotech – Roles, Skills & How to Start
A Contract Research Organization (CRO) offers significant support to Pharmaceutical, Biotechnology, and Medical Device Industries by providing outsourced research services. As global demand for drug development and clinical trials accelerates, CROs have become one of the most promising platforms for launching or growing a career in Biotechnology. Whether you are a Life Science graduate, a data enthusiast, or a healthcare professional, a CRO career in Biotechnology offers diverse roles according to your interests and strengths.
In this article, we’ll explore what a CRO career in Biotech is, the skills you need, and the job roles available so that you can take the first confident step toward this rewarding industry.
What is a CRO?
A Contract Research Organization (CRO) provides outsourced support to Pharmaceutical, Biotechnology, and medical device companies, offering services such as clinical trials, regulatory affairs, and data management. A Contract Research Organization helps industries conduct research without requiring in-house infrastructure. Top notch companies are IQVIA, ICON, GSK, Indegene, Parexel and many more, but also many startups also work with CROs, making startups also work with CROs, making it a growing field for job seekers.
Why Choose a CRO Career in Biotech?
CRO careers offer fast-paced, worldwide exposure to real-world Biotechnology projects. You will gain hands-on exposure across various therapeutic areas as well as tools, and also work with pharma clients. The career path tends to grow faster than in academia and offers valuable cross-functional learning.
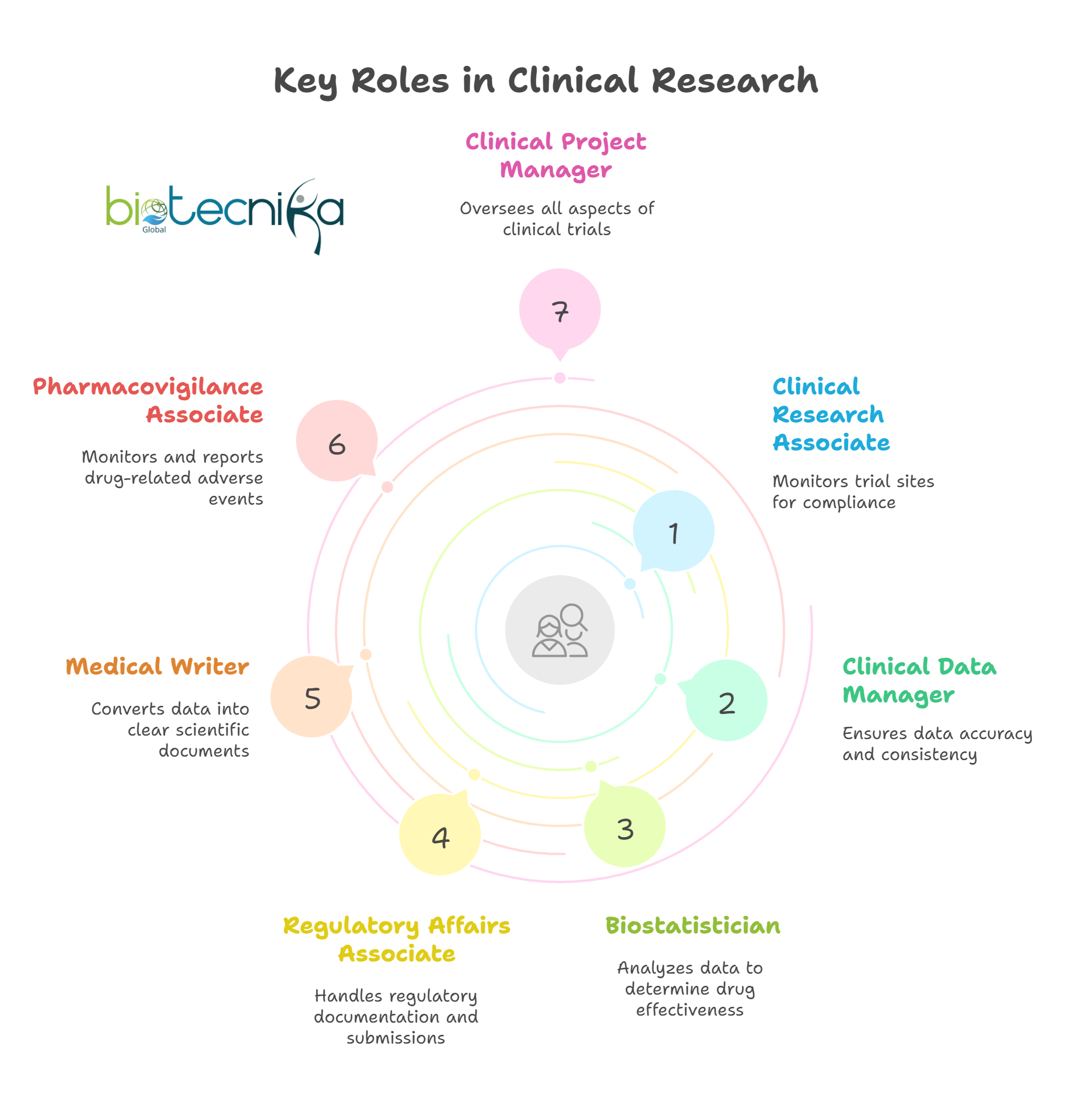
Top Job Roles in a CRO – In Short Paragraphs
1. Clinical Research Associate (CRA)
CRAs play an important role in monitoring clinical trial sites to ensure compliance with protocols, ethical standards, and regulatory guidelines. They play a major in verifying data accuracy and ensuring the trial runs smoothly. Excellent communication skills, travel readiness, and knowledge of Good Clinical Practice are essential.
2. Clinical Data Manager
These roles include managing clinical trial data, ensuring its accuracy, consistency, and adherence to compliance standards. This role plays an important role in delivering clean and reliable data that supports regulatory submissions and decision-making.
3. Biostatistician
They work as trial methodologies as well as analyze clinical data to determine drug effectiveness and safety. Biostatisticians use many tools such as the Statistical Analysis System (SAS) and the R programming language to perform statistical evaluations, which is important for drawing scientific conclusions from the complex data.
4. Regulatory Affairs Associate
These role play an important role in handling all documentation and submissions required by regulatory agencies such as the Food and Drug Administration (FDA) or the European Medicines Agency (EMA). Their work ensures clinical trials and drug development processes meet all legal and safety requirements across global markets.
5. Medical Writer
They major role in converting complex clinical and scientific data into clear, accurate documents like study protocols, Clinical Study Reports (CSRs), as well as journal and articles. The Medical Writer role requires a strong scientific understanding and excellent writing skills.
6. Pharmacovigilance Associate
They focus on monitoring and reporting drug-related adverse events during and even after clinical trials. Pharmacovigilance Associate analyzes the safety of the data to ensure patient protection and regulatory compliance, using tools like Argus or Oracle.
7. Clinical Project Manager
Project Managers oversee all aspects of clinical trials, from budgeting and scheduling to team management. They ensure that projects meet timelines, stay within budget, and comply with regulatory standards, making them key to trial success.
Essential Skills to Succeed in a CRO – In Short Paragraphs
Technical Skills
To build a CRO career in Biotech, you need to understand clinical trial protocols, Good Clinical Practice, International Conference on Harmonisation, and the use of Electronic Data Capture systems. Familiarity with regulatory documentation processes is also essential for both accuracy and compliance.
Soft Skills
Strong communication, time management, and teamwork are vital.CRO career in Biotech environments are fast-paced and collaborative, so problem-solving and attention to detail help professionals adapt and succeed.
Digital & Analytical Tools
Depending on your role, you may need tools like SAS, R, or Python for data roles, or MedDRA and WHO Drug for PV and regulatory work. Proficiency in Excel and CTMS platforms also adds significant value.
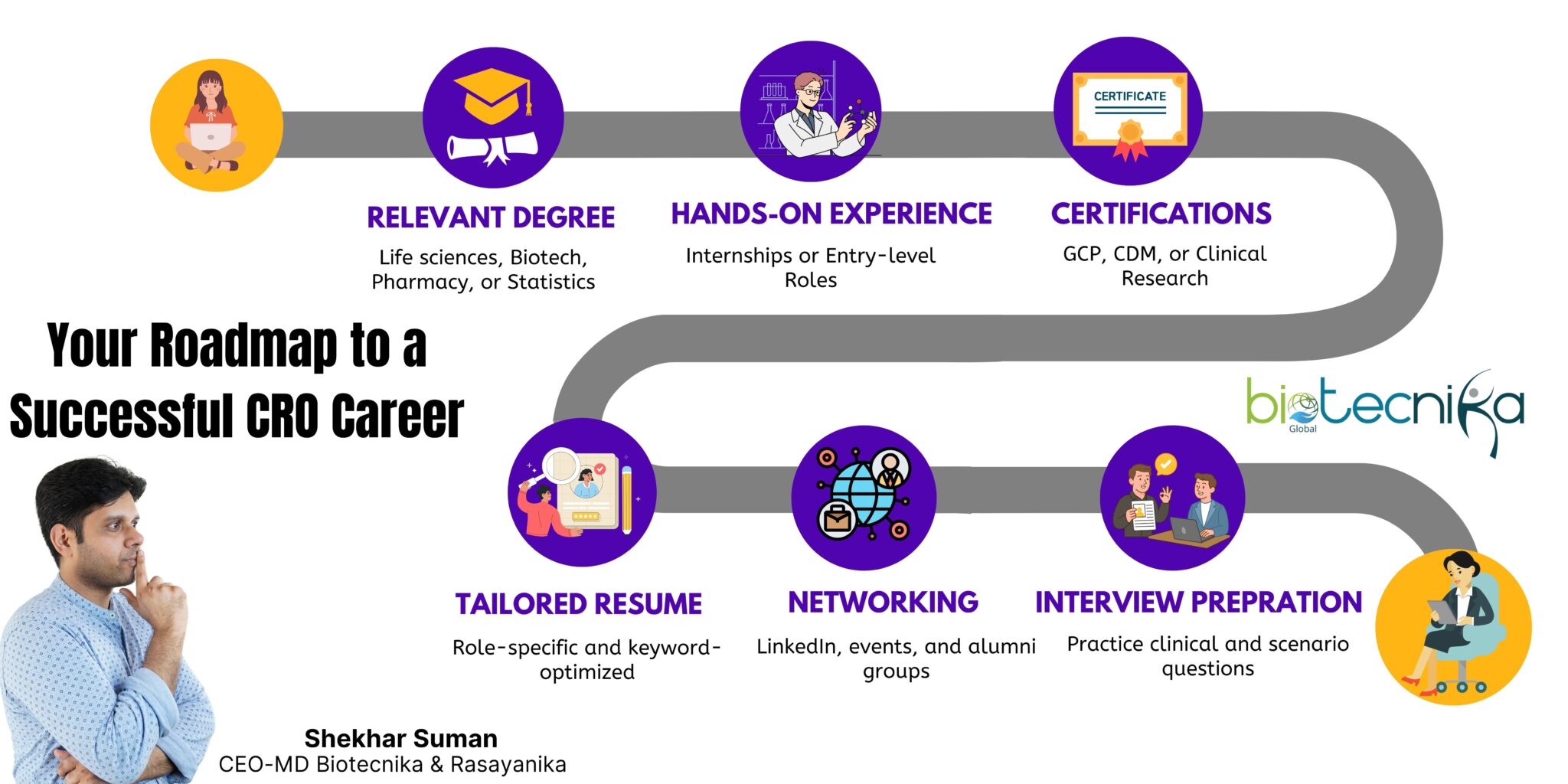
How to Get Started in a CRO
1. Build a Strong Educational Foundation
The right academic background is essential to start a career in any particular field. Most CRO roles require a degree in a Life Science, Biotechnology, Pharmacy, or statistics field. This foundational education will provide you with the essential knowledge of Human Biology, Clinical Research processes, and data interpretation.
A solid academic base not only makes you eligible for various roles but also helps you to understand the core responsibilities involved in Clinical Trials, Regulatory Processes, as well as Drug Development.
- Apply for Internships or Entry-Level Jobs
Gaining hands-on experience is a crucial step in breaking into a CRO. Entry-level roles like Clinical Trial Assistant, Data Coordinator, or Pharmacovigilance Trainee are ideal starting points. This role provides practical exposure to the daily operations of activities.
Even short-term internships can make a difference by allowing you to apply your theoretical knowledge, understand CRO workflows, and develop a network of professional contacts.
- Get Certified
Certifications are significantly important to boost your employability in the CRO industry. They showcase that you are industry-ready and have up-to-date knowledge of the regulatory and ethical standards expected in clinical research. Courses in Good Clinical Practice (GCP), Clinical Research Methodologies, Pharmacovigilance, or Clinical Data Management (CDM) are highly valued. There are many platform that provides these courses, such as Biotecnika.
These certifications often cover practical and regulatory frameworks, which help bridge the gap between academic knowledge and real-world application, making you a more competitive candidate.
- Customize Your Resume and Cover Letter
A generic resume won’t get you far in this competitive field. It’s essential to tailor your resume and cover letter to each CRO role you apply for. Highlight your academic background, relevant skills, certifications, and any hands-on experience, including projects or internships.
Use specific keywords from the job description to align your applications with what the employer is looking for. A well-crafted application or resume that showcases your detail and clear understanding of the role can increase your chances of getting shortlisted.
- Network Strategically
In the CRO industry, networking can play an important role. Building connections with professionals through LinkedIn, conferences, webinars, industry events, or alumni groups can open doors to job opportunities that aren’t advertised publicly.
Being active in professional communities helps you to stay updated on the latest trends, tools, and hiring needs in the clinical research field. This connection often leads to referrals, which can fast-track your entry into the industry.
- Practice Interview Scenarios
Preparation plays a key role once you start giving interviews. CRO interviews often involve questions on clinical trial phases, regulatory compliance, data quality, and ethical considerations. Sometimes, you may also face scenario-based questions where you will need to explain how you will handle real-world challenges.
Practicing these kinds of questions helps you answer more confidently and effectively. It’s also a good idea to stay updated on industry developments so you can bring relevant insights into the conversation.
Working in a Contract Research Organization is not just a job; it is a dynamic, long-term career opportunity in Biotechnology. As you gain experience, you can become a master in diverse fields such as Oncology, Digital Therapeutics, or Global Trial Coordination. They are opportunities to rise into leadership roles, such as Clinical Project Manager or Regulatory Affairs Director. If you are aiming for fast-paced learning, meaningful impact, and steady growth, a CRO career in Biotech could be your ideal gateway into Biotech and the pharmaceutical Industry.