Quality Assurance in Biotechnology: The Future of QA in the Global Biotech Industry
In the research lab of London, fluorescent lights hummed softly over the rows of incubators in the middle of the night. The team of researchers working on this project was excited by this success. They had just developed a revolutionary therapy that holds the power to rewrite the future of patients suffering from a rare genetic disorder. But one member of the team was not celebrating.
Scrolling through the data with a trained, unrelenting eye, a QA specialist noticed something odd. A tiny anomaly buried in thousands of test results. Most would have overlooked it. But this specialist knew the stakes. When they dug deeper, they uncovered a contamination risk that could have caused serious side effects if the therapy had been administered to patients. One call, one corrective action, and a potential disaster was averted.
This moment, invisible to the outside world, perfectly illustrates the heartbeat of biotechnology: innovation without quality is dangerous. Behind every life-saving breakthrough are the vigilant QA professionals, ensuring that science does not just push boundaries. It does so safely, reliably, and globally.
Why Quality Assurance in Biotechnology Is More Crucial Than Ever
With the advancing technology, Biotechnology is growing faster than ever before. From gene therapies to personalized vaccines, the biotech industry is rewriting the future of medicine, agriculture, and real-world problems.
Research labs are giving birth to new innovations every day across the globe, but these innovations are often complex. The products are more intricate. The regulations are stricter, and patient expectations are higher than ever.
In the complex scientific world, a single quality failure can lead to recalls, lawsuits, or even harm patients. A Quality Assurance Analysts make sure that every process, from research lab to clinical trials and manufacturing, meets the highest standards of safety and effectiveness.
They are not just inspectors. They are proactive guardians who prevent mistakes even before they happen. In the international biotech industry, where trust is everything, QA is the backbone of credibility.
The Evolving Role of Quality Assurance in Biotechnology Professionals
With the word “evolution”, don’t be tense that we will talk about history. We will just have a look at the Quality Assurance in Biotechnology before and now. So, traditionally, QA Analysts were more focused on detecting defects and ensuring compliance at the end of the production line. Today, their role in the industry is beyond that. They are now in a strategic, business-aligned function that influences innovation from start to finish.
-
From Quality Control to Quality Engineering
You must have heard about Quality Control, but do you know what Quality Engineering is? Well, in simple words, it is about ensuring the quality of the product at every stage. You don’t have to wait until the product is finished. The QA professionals collaborate with research, development, and manufacturing departments to design every process to minimize risk and optimize the outcomes.
-
Business-Aligned Quality Assurance in Biotechnology
Quality Assurance is no longer confined to a single department. Today’s professionals are expected to grasp business priorities, market shifts, and regulatory demands. When quality is aligned with the overall strategy, it not only safeguards patients. It also drives sustainable growth for the organization.
Key Drivers Shaping the Future of Quality Assurance in Biotechnology
Currently, there are several trends that are reshaping QA. If you want to enter the QA world, remember, these trends can be both challenging and also the best opportunities you can get.
1. Technological Innovation
The world has got two new superpowers, Artificial Intelligence (AI) and Machine Learning (ML). These superpowers are now revolutionizing the QA. They analyze massive datasets, detect patterns, predict quality risks, and automate repetitive tasks. And do you know what the result is? You will have faster, more accurate, and proactive QA processes, with reduced error rate.
2. Digital Platforms and Cloud Solutions
Cloud-based Quality Management Systems (QMS) are transforming how global biotech teams operate. Real-time monitoring, remote collaboration, and instant access to quality metrics allow teams to respond quickly to issues, no matter where they are located.
3. Advanced Analytics
With the help of predictive analytics and data visualization, QA teams can spot patterns early, foresee potential risks, and take corrective action before problems affect the product. This makes the entire QA process more proactive, efficient, and strategic.
Regulatory Evolution and Global Harmonization
As you know, biotech products travel across the world. The QA teams face the challenge of managing diverse and complex regulations.
-
Risk-Based Quality Management (RBQM)
This focuses on identifying potential risks throughout the product lifecycle and mitigating them proactively.
-
Global Harmonization for Quality Assurance in Biotechnology
It ensures consistency across markets, reducing delays and compliance challenges for international biotech companies.
-
Data Integrity Compliance
It is vital, with regulations like 21 CFR Part 11 governing electronic records and signatures. Ensuring accurate, traceable data is now a non-negotiable aspect of QA.
These changes in regulations show that QA is now indispensable. They play a key role in the success and trustworthiness of biotech companies around the world.
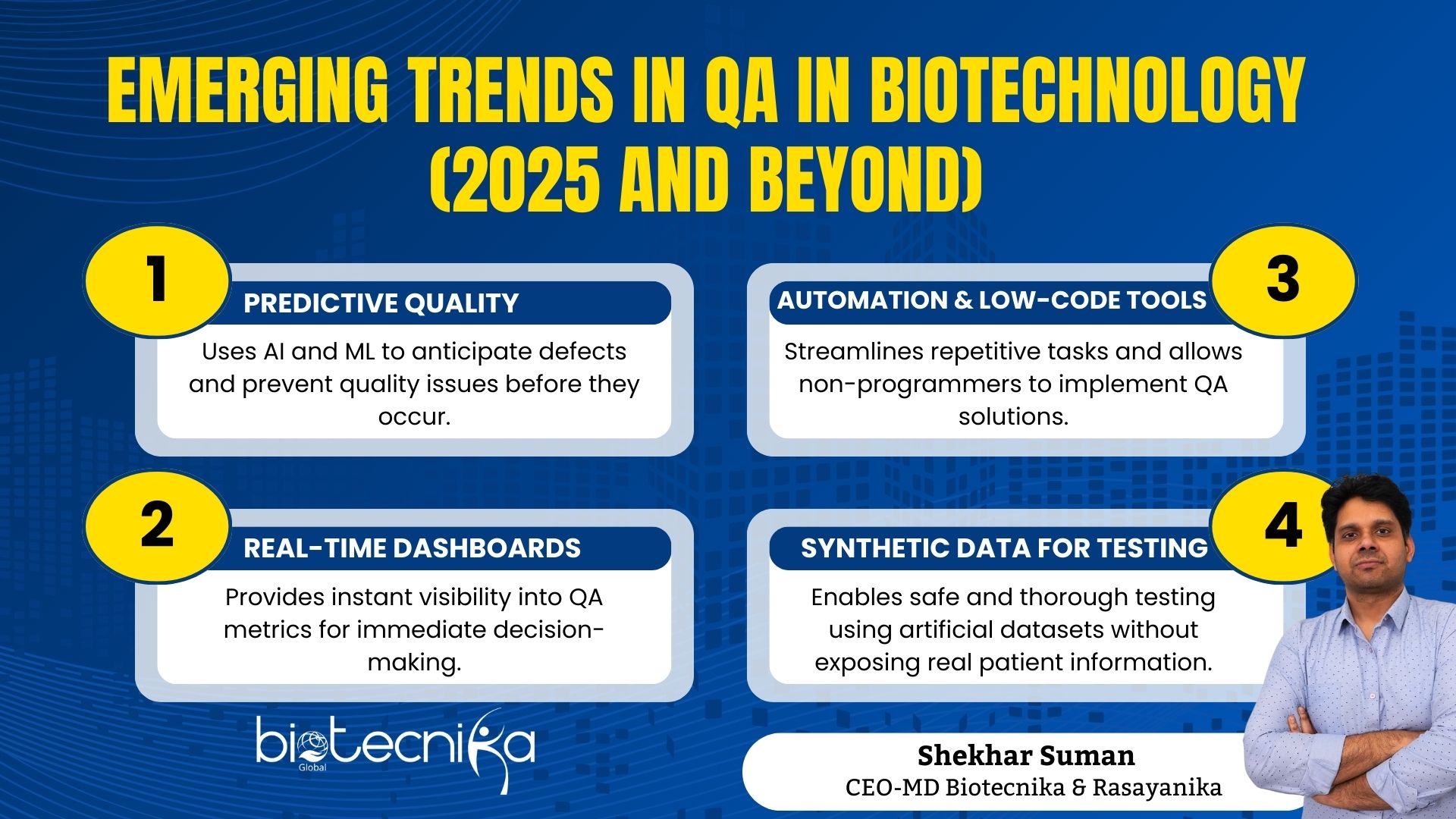
Emerging Trends Quality Assurance in Biotechnology for 2025 and Beyond
The future of QA is more than just catching errors. It is all about anticipating issues, driving innovation, and making sure top-quality products are provided to the public.
-
Predictive Quality
With modern technology, AI, and ML, we can predict the quality of products. This helps the term in identifying potential defects before they occur. By identifying issues, the biotech companies can work on them and take the right action. This will prevent failures.
-
Real-Time Dashboards
We know how difficult continuous monitoring can be. Now we have real-time dashboards that will provide instant insights into quality metrics. This will help QA teams act more quickly. This visibility helps the team improve both efficiency and safety.
-
Automation and Low-Code Tools
We all want our work to be done automatically, especially when you have a high-end task. Automation helps us reduce repetitive manual tasks. And now QA professionals don’t have coding knowledge to handle these tasks. They can do it with low-code/no-code platforms to create effective solutions. These tools democratize QA and increase operational efficiency.
-
Synthetic Data for Testing
Using synthetic data lets researchers test thoroughly while keeping patient information private. This method is particularly useful for clinical trials, meeting regulatory requirements, and evaluating new algorithms.
Impact of Emerging Technologies
Several emerging technologies are poised to transform Quality Assurance in Biotechnology:
-
Blockchain
It ensures secure and transparent tracking across the supply chain. It also improves accountability and helps in meeting regulatory standards.
-
Advanced Automation Tools
These tools allow test cases to be created and executed more efficiently. They reduce manual work while increasing test coverage.
-
TestOps Practices
These practices combine both QA and agile development methods. It supports continuous testing and faster release cycles.
Challenges and Opportunities for Quality Assurance in Biotechnology Professionals
| Challenge / Opportunity | Description | How to Overcome / Leverage |
| Keeping up with rapid technological advances | QA professionals must adapt to AI, automation, cloud QMS, predictive analytics, and emerging tools. | Continuous learning, online courses, certifications in AI, data analytics, and digital QA platforms. |
| Navigating complex global regulations | Biotech QA requires understanding multiple regulatory frameworks (FDA, EMA, ICH) and harmonization across countries. | Gain regulatory knowledge through training programs, workshops, and international QA guidelines. |
| Maintaining data integrity in digital environments | Increasing reliance on electronic records and cloud systems heightens the risk of errors, fraud, or data loss. | Follow best practices for electronic documentation, audits, and compliance with 21 CFR Part 11 and related standards. |
| Career growth in quality engineering, regulatory affairs, and data analytics | QA is evolving into a strategic, high-impact function with diverse career paths. | Build cross-functional skills, learn emerging QA technologies, and network with professionals in biotech QA. |
| Global collaboration and exposure | QA professionals work with teams worldwide, requiring adaptability and communication across cultures. | Develop soft skills, multilingual communication abilities, and familiarity with global quality standards. |
| Direct impact on patient safety and trust | QA ensures that biotech products are safe, effective, and trustworthy, affecting real patient outcomes. | Embrace a proactive mindset, focus on risk management, and use predictive tools to prevent quality issues. |
Conclusion
The biotech industry is moving fast, and quality assurance is keeping pace. For students and recent graduates, a career in QA offers exciting opportunities. By learning new technologies, understanding regulations, and sharpening analytical and problem-solving skills, future QA professionals can play a key role in delivering safe and innovative therapies to patients worldwide.
The story of that late-night QA check in Boston shows that behind every scientific breakthrough, there’s a careful, vigilant mind making sure it’s done safely. As biotechnology continues to change healthcare around the world, QA will remain central to innovation, helping bring life-saving products to those who need them most.