Global Career In Regulatory Affairs – Booming Life Sciences Sector
Regulatory affairs is one of the most crucial fields in the life sciences industry, ensuring that healthcare products such as pharmaceuticals, medical devices, biologics, and cosmetics comply with national and international regulations. As global markets continue to expand, the need for trained regulatory professionals who understand diverse regulatory systems is increasing rapidly. Building a global career in regulatory affairs means working at the intersection of science, law, and policy, with opportunities to make a real impact on public health worldwide.
This article explores the educational backgrounds suited for regulatory roles, career pathways, certifications, and global job markets to help you take the first step or advance further in this in-demand profession.
- What Is Regulatory Affairs?
Regulatory affairs is a field that ensures Biotech and Pharma companies comply with all regulations and laws pertaining to their business. In the Life Science industry, this primarily involves Pharmaceutical, Biotechnology, Medical Devices, as well as Healthcare products. Regulatory Affairs professionals act as the link between regulatory agencies and their organizations. They manage submissions, licenses, and approvals needed for the product to enter and stay in global markets.
- Why Choose a Global Career in Regulatory Affairs?
A global career in regulatory affairs allows you to work with diverse regulatory systems, cultures, and markets. Professionals in this field often deal with the US FDA, European Medicines Agency (EMA), Health Canada, PMDA (Japan), and other global regulatory bodies. This exposure broadens your skill set, enhances your professional credibility, and opens new opportunities to international jobs.
Additionally, as companies expand their markets across borders, they seek regulatory professionals who understand global guidelines and can help them achieve faster. Market entry with fewer compliance issues.
- Educational Background Required
- Science or Pharmacy Degrees – Most regulatory affairs professionals come from a background in Pharmacy, Life Sciences, Biotechnology, Chemistry, or Medicine. A bachelor’s degree in any of these fields is typically the minimum requirement to enter the profession.
- Postgraduate Qualifications – Having a Master’s degree in Regulatory Affairs or a related field will significantly increase your profile. Many institutions now offer specialized postgraduate programs that focus on Global Regulations, Clinical Trials, and Regulatory Strategy.
- Key Skills You Need
- Regulatory Knowledge – Understanding ICH guidelines, FDA regulations, EMA procedures, and other country-specific regulatory pathways is critical. This knowledge is important for drafting and submitting dossiers, ensuring product safety, and communicating with health authorities.
- Communication and Documentation – Since a large part of the job involves documentation and correspondence with regulatory bodies, excellent written and verbal communication skills are essential.
- Analytical Thinking – You need the ability to interpret complex regulations, scientific data, and trial results, and apply them to real-world business decisions.
- Project Management – Regulatory Affairs professionals often manage multiple submission timelines and coordinate between departments. Strong organizational as well as project management skills are highly valued.
- Entry-Level Pathways
- Internships and Trainee Positions – For fresh graduates, internships or RA trainee roles in pharmaceutical companies or contract research organizations (CROs) are the best way to gain practical experience. These roles provide hands-on experience with regulatory processes and submission documentation.
- Clinical Research and Quality Assurance – Some professionals enter regulatory affairs after working in clinical research, quality assurance, or medical writing. These roles provide foundational knowledge of industry regulations and standards.
- Building Experience and Climbing the Ladder
- Regulatory Associate and Specialist Roles – With 1 to 3 years of experience, professionals often advance to roles like Regulatory Affairs Associate or Regulatory Affairs Specialist. These positions involve preparing regulatory submissions, managing timelines, and supporting the development of new products.
- Managerial and Strategic Roles – As you gain experience (5–10 years), you may move into regulatory manager, regional RA lead, or director-level roles. Here, your responsibilities will expand to include strategic decision-making, global regulatory planning, and team leadership.
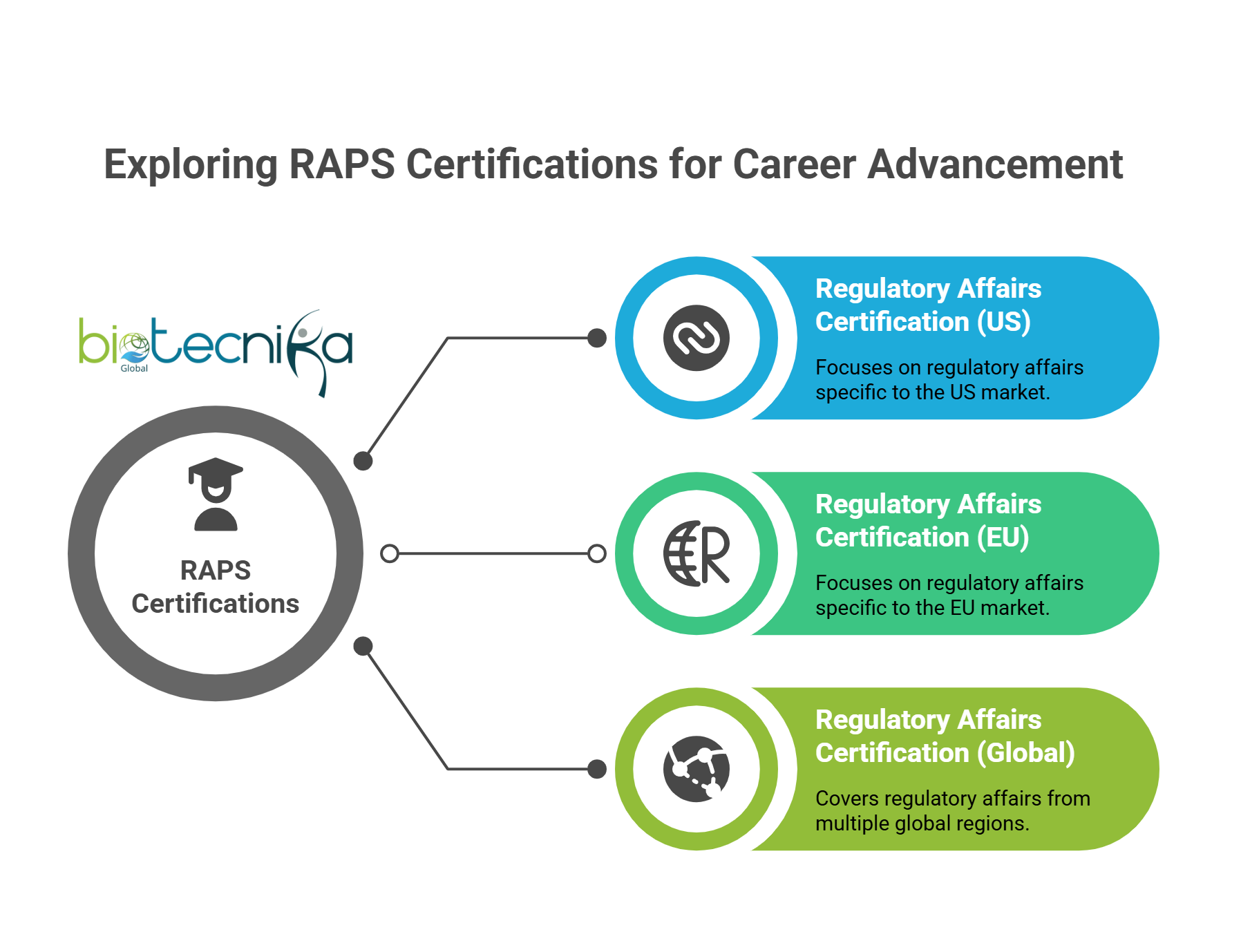
- Certifications to Boost Your Global Career
- RAPS Certifications – The Regulatory Affairs Professionals Society (RAPS) offers globally recognized certifications, such as:
-
- Regulatory Affairs Certification (US) – Focuses on US regulations.
- Regulatory Affairs Certification (EU) – Focuses on European regulations.
- Regulatory Affairs Certification (Global) – It covers regulations from multiple regions.
These certifications validate your expertise and increase your credibility in global markets.
- Postgraduate Diplomas and Online Courses – Many universities and platforms, such as Biotecnika and FutureLearn, offer postgraduate diplomas and certifications in regulatory affairs, often focusing on global standards. Grabbing such a course certificate helps to bridge the gap between academic knowledge and industry requirements, especially for those transitioning from other roles.
- Understanding Global Regulatory Systems
- United States – FDA – The Food and Drug Administration regulates drugs, biologics, and medical devices in the US. Professionals working with the FDA must understand the IND, NDA, BLA, and 510(k) submission processes.
- European Union – EMA – The European Medicines Agency oversees drug approval across EU countries. RA professionals should be familiar with centralized, decentralized, and mutual recognition procedures.
- Asia-Pacific Markets – Countries such as Japan, China, South Korea, and India have robust regulatory ecosystems. Knowledge of local regulatory bodies like PMDA (Japan), NMPA (China), and CDSCO (India) is essential for a global career.
- Opportunities Across Industries
- Pharmaceuticals and Biologics – The majority of RA professionals work in pharmaceutical and biotech companies, handling everything from pre-approval documentation to post-marketing surveillance.
- Medical Devices – RA specialists in this sector manage device classifications, technical files, CE markings, and compliance with ISO standards.
- Nutraceuticals and Cosmetics – These emerging sectors also require compliance professionals due to evolving global regulations around health claims, ingredients, and product safety.
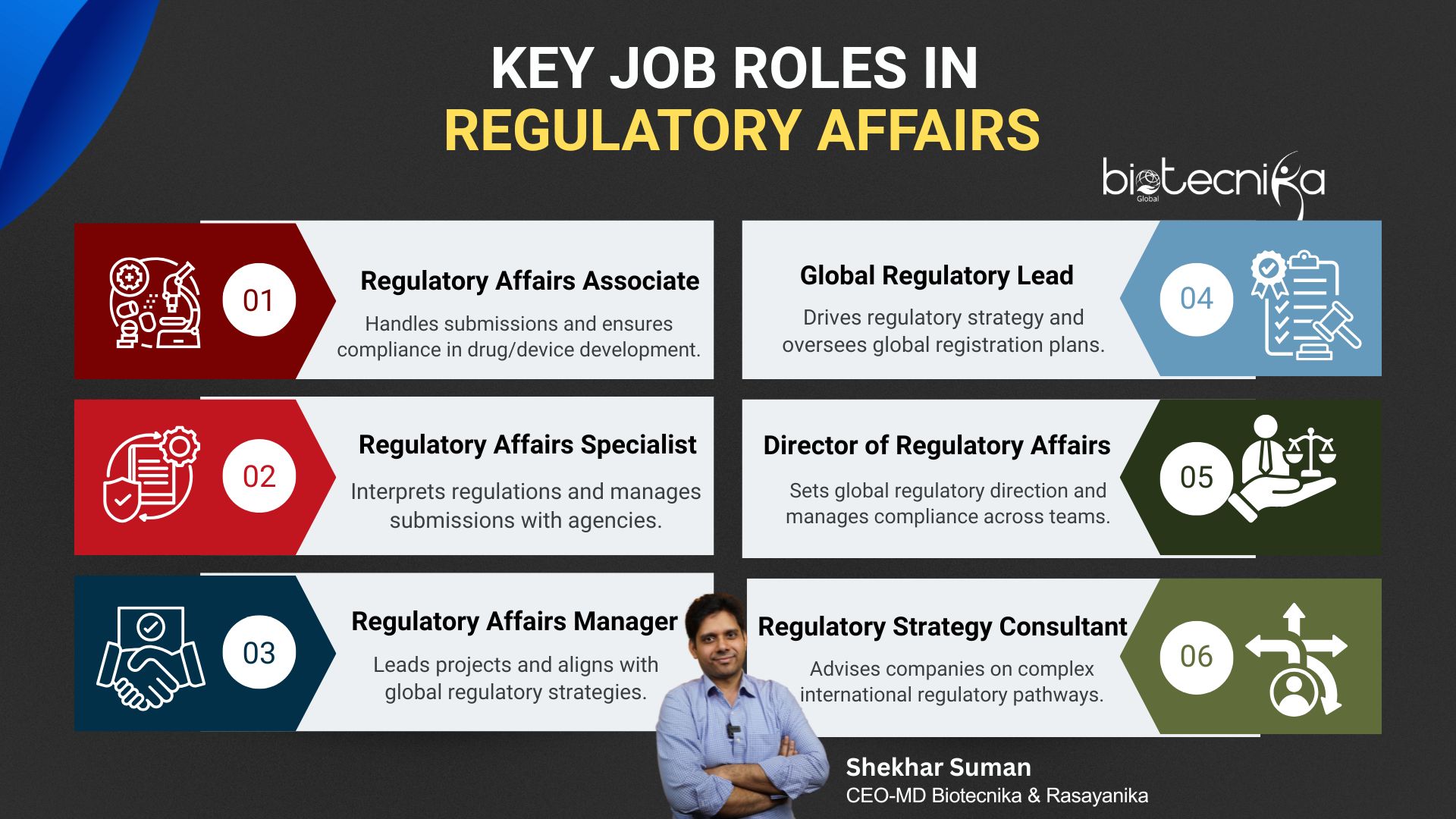
- Job Titles to Look For
- Regulatory Affairs Associate – Entry-level role focused on preparing and submitting regulatory documents. Supports compliance and documentation tasks in the development of drugs, devices, or biologics.
- Regulatory Affairs Specialist – Mid-level position responsible for interpreting regulations, coordinating submissions, and liaising with regulatory bodies. Ensures product compliance throughout development.
- Regulatory Affairs Manager – Collaborates with oversight of Regulatory Projects and teams. Manages and supervises the timely submission to ensure alignment with global regulatory strategies.
- Global Regulatory Lead – Leads regulatory strategy across multiple countries. Works closely with cross-functional teams to develop and execute global registration plans.
- Director of Regulatory Affairs – Senior leadership role plays a vital role in global regulatory strategy, compliance, and interfacing with health authorities. Directs teams and supports business goals.
- Regulatory Strategy Consultant – Independent or firm-based expert offering strategic advice to companies on navigating complex regulatory pathways across global markets
These roles are found across:
- Multinational pharmaceutical and biotechnology companies
- Contract Research Organizations (CROs)
- Regulatory consultancies
- National and international health authorities
Every job role plays a vital role in enabling global compliance and market access. For anyone aiming to build an international career in regulatory affairs, understanding these job roles can help find the right career path. By
A global career in Regulatory Affairs is both impactful and dynamic. Whether you are helping launch a life-saving cancer drug or ensuring the safety of a new medical device, your research work influences public health on a global scale. By enrolling and gaining the right education, certificates, as well as worldwide exposure, you can build a successful career in this fast-growing domain.
With healthcare regulations constantly evolving and more countries aligning with international standards, now is the perfect time to pursue a global career in regulatory affairs.