CROs in Clinical Trials jobs – Roles, Services & Job Opportunities
Clinical trials are the foundation of evidence-based medicine. They help researchers determine whether a new drug, therapy, or medical device is both safe and practical. However, bringing a product from concept to market involves multiple trial phases, extensive planning, and strict regulatory compliance, which can overwhelm the Pharmaceutical and Biotechnology industries. This is where Contract Research Organizations (CROs) step in.
CROs are specialized service providers that help sponsors conduct clinical trials more efficiently and effectively. From designing early-stage trials to collecting real-world data post-approval, CROs are essential to clinical research success. They are also key employers in the life sciences sector, making CROs in clinical trials jobs an attractive career path for professionals seeking dynamic, global, and meaningful work.
What is a CRO?
A Contract Research Organization (CRO) is a team that provides outsourced clinical research services. These services support the Pharmaceutical, Biotechnology, and medical device industries in conducting research and clinical trials. CROs can operate on a national or global scale, offering full-service or functional service capabilities.
CROs offer:
- Clinical Trial Design and Planning – CRO helps sponsors create a scientifically sound trial plan. This includes selecting research endpoints, determining sample sizes, writing protocols, and planning the overall strategy. A well-designed trial ensures reliable results and faster regulatory approvals.
- Patient Recruitment and Retention – CROs identify eligible participants as well as implement strategies to enroll them more efficiently. They also manage communication, follow-up, and engagement to ensure participants remain in the study, minimizing dropouts and delays.
- Regulatory Compliance Support – CROs navigate complex global regulations, including those from the Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). They prepare and submit the required documents, communicate with regulatory authorities, and ensure that trials adhere to Good Clinical Practice standards.
- Monitoring and Data Collection – CROs oversee trial sites to ensure procedures are followed correctly. They monitor patient safety, collect data using an electronic system, and check for protocol deviations to ensure data integrity.
- Statistical Analysis and Reporting – CRO statisticians analyze trial data to determine the safety and efficacy of the study. They generate statistical reports, interim analyses, and support submissions with valid, scientifically backed conclusions.
- Medical Writing and Documentation – CROs create important documents, including clinical study reports (CSRs), investigator brochures, informed consent forms, and regulatory submissions. These are essential for transparency, compliance, and approval.
CROs in clinical trials jobs include a wide range of roles such as Clinical Research Associates (CRAs), Clinical Data Managers, Regulatory Affairs Specialists, Biostatisticians, and Project Managers.
CRO Involvement in Clinical Trial Phases
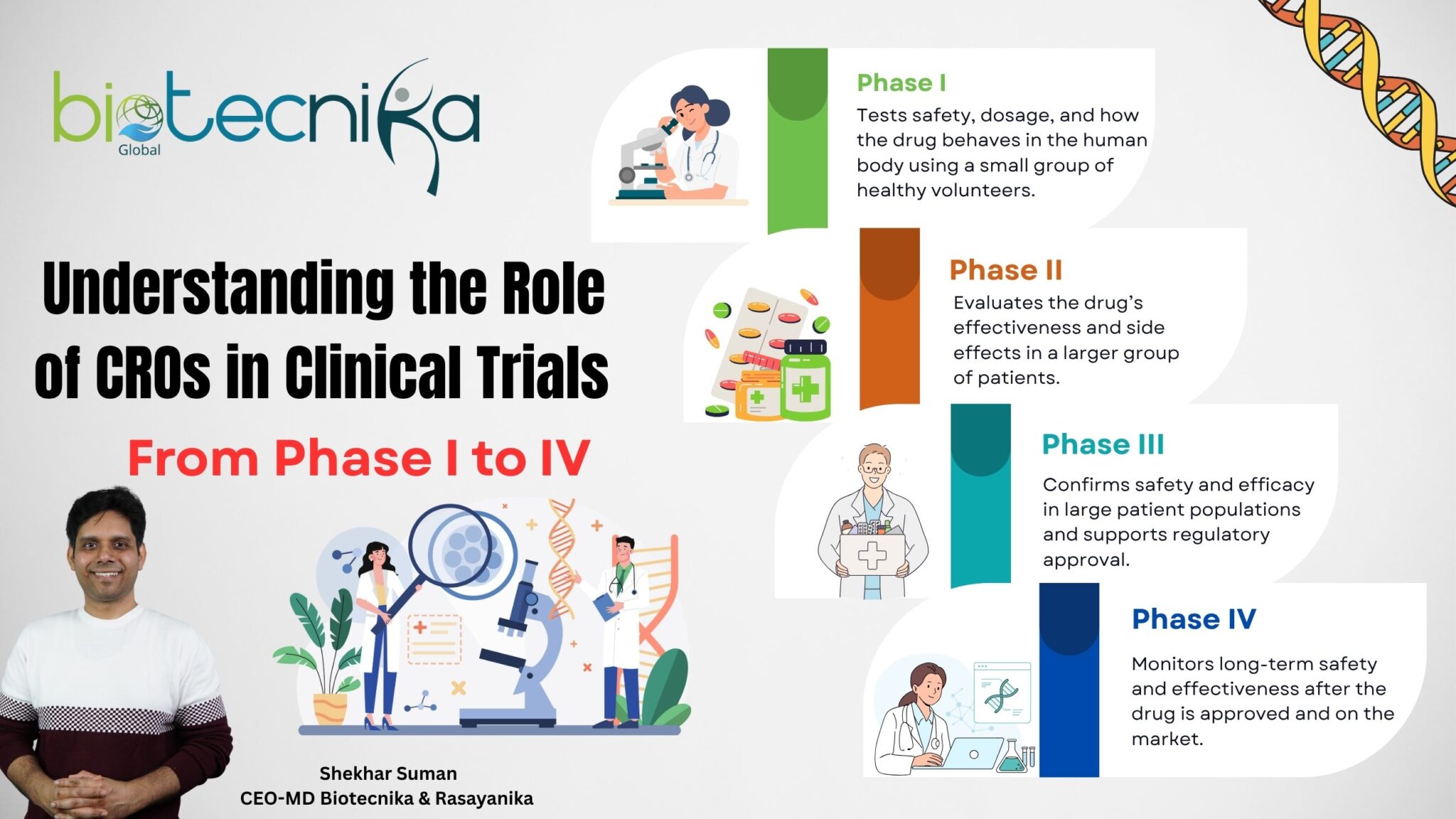
Phase I: First-in-Human Studies
Objective: To evaluate safety, dosage levels, and how the drug behaves in the human body.
CRO’s Role: In Phase I trials, CROs design the study, select suitable volunteers, monitor patient safety in real-time, and manage early data analysis. Their goal is to evaluate how the drug works in the human body and determine safe dosage levels. This phase provides foundational experience for professionals entering the field of clinical trials, particularly in roles such as trial coordination, laboratory monitoring, and early-stage data management.
Phase II: Efficacy and Dose-Response
Objective: To assess the treatment’s effectiveness and further evaluate safety.
CRO’s Role: CROs lead protocol development, train investigators, ensure accurate safety reporting, and perform interim data analyses. These trials test the treatment’s effectiveness, as well as helping in refining dosing. Phase II trials require expertise in pharmacovigilance, analytics ,and trial operations, providing key opportunities in CRPs in Clinical trials jobs focused on Data Science and Clinical development.
Phase III: Confirmatory Trials
Objective: To confirm safety and efficacy in large patient populations.
CRO’s Role: CROs coordinate large-scale trials, manage digital recruitment strategies, oversee the Electronic Data Capture (EDC) system, and prepare regulatory submissions. With thousands of patients and high complexity, Phase II demands experienced professionals, which enhances the growth in CROs in Clinical Trials jobs, such as Trial Monitors, Biostatisticians, and Regulatory Managers.
Phase IV: Post-Marketing Surveillance
Objective: To monitor long-term safety and effectiveness.
CRO’s Role: After drug approval, CROs conduct real-world evidence studies, operate Pharmacovigilance system, and perform Health Economics and Outcomes Research (HEOR). These roles are essential for monitoring long-term safety, effectiveness, and offering specialized career paths in CROs in Clinical Trials jobs focused on post-market analysis and patient outcomes.
Types of CRO Services Offered
1. Full-Service CROs: Manage the Clinical Trial lifecycle, offering comprehensive services. These organizations typically offer a wide variety of CROs in entire clinical trials jobs, from project managers to quality assurance specialists.
2. Functional Service Providers (FSPs): CROs focus on one or two services, like Clinical Monitoring or Data Management. FSPs provide focused CROs in clinical trials jobs that suit professionals wanting to specialize.
3. Specialty CROs: Offer niche services in specific therapeutic areas or regions. These CROs create highly focused career paths for people passionate about a certain disease area or demographic.
4. Virtual CROs: Rely on digital tools and remote trial designs. They provide innovative CROs in clinical trials jobs such as decentralized trial coordinators, virtual trial designers, and telemedicine experts.
CROs and Emerging Technologies – Modern CROs leverage technology to streamline clinical trials and attract top talent. Key advancements include:
- AI/ML – Artificial Intelligence and Machine Learning help identify eligible trial participants faster by analyzing health records and historical data, speeding up recruitment and improving outcomes.
- Wearables and Digital Health Tools – Devices such as smartwatches as well as health apps, collect real-time data remotely, reducing the need for site visits and enhancing patient monitoring.
- Blockchain – Blockchain ensures secure, transparent, and tamper-proof storage of trial data and consent records, boosting trust and compliance.
- Decentralized trial platforms – Virtual tools, such as telemedicine and eConsent, enable trials to run remotely, increasing patient access and facilitating global participation.
Tech-driven transformation has led to a boom in CROs in clinical trials jobs, particularly in data science, AI model validation, and tech integration roles.
Regulatory and Ethical Considerations
CROs operate under strict ethical and regulatory frameworks, ensuring patient rights and scientific integrity are recorded. This creates career opportunities in:
- GCP and compliance auditing
- IRB/EC documentation and ethics reviews
- Informed consent and patient education
For professionals seeking roles that bridge ethics and science, CROs in clinical trials offer fulfilling options.
Top Global CROs Offering Clinical Trials Jobs
- IQVIA – A global leader known for its vast job openings in data analytics and trial operations.
- Labcorp Drug Development – Offers roles in central lab services, bioanalysis, and monitoring.
- Parexel – Known for regulatory consulting and medical communications roles.
- Syneos Health – Combines clinical trial and commercial services, creating hybrid job opportunities.
- ICON plc – Offers a wide range of therapeutic-area specific roles.
- Medpace – Recognized for a hands-on, investigator-led clinical model, with jobs in project management and regulatory writing.
All of these companies consistently post thousands of CROs in clinical trials jobs across the globe.
CROs are vital not only for the success of Clinical Trials but also for shaping the clinical research job market. With their growing influence in trial design, global execution, and tech integration, they are key employers in the Life Sciences space.
Whether you are a recent graduate, a seasoned researcher, or a healthcare professional seeking industrial exposure, CROs in clinical research trials offer meaningful, high-impact, and future-ready career opportunities.