How CROs Are Accelerating Drug Discovery in 2025 – Trends and Innovations
A scientist in the United States analyzes AI-generated Molecular data from a Scientist in India. A patient in Africa records his symptoms on a Medical wearable device, which is then instantly registered on a Clinical Trial dashboard for Researchers in Japan. Isn’t this amazing? But what’s the conclusion? There are no clipboards or lab coats, but just digital science, connected in action globally.
Welcome to the new era of Drug Discovery, where time zones are no longer a barrier and innovation flows freely across national borders. And do you know what’s at the center of this revolution? It is the CROs (Contract Research Organizations). They were once behind-the-scenes Scientific executors, but now their innovation engines drive Pharmaceutical advancements and transformation worldwide.
CROs accelerating Drug Discovery are leading the Research sector from the front. From decentralized trials to Green Chemistry and AI-driven analytics, they exponentially accelerate Drug Development.
But what ignited this revolution? What mindsets, Global shifts, and technologies redefined their role worldwide? Read the article further to find these answers and to understand the innovations, advancements, and trends in CROs.
Step into the future of Healthcare and Medicines, where CROs lead the advancements and technological revolution!
The Evolution
From being transactional vendors to being strategic collaborators, the transformative evolution of CROs is a trend in the Healthcare and Biopharmaceutical sectors. It was earlier confined to compiling Regulatory documents, providing Research services, or managing Clinical Trials. Still, CROs are now actively participating in driving innovation as well as reshaping Research strategies in Drug Development. This dramatic shift reflects the increased complexities of Drug Discovery as well as the urgent requirement for efficient and integrated solutions that ultimately accelerate timelines as well as optimize resources.
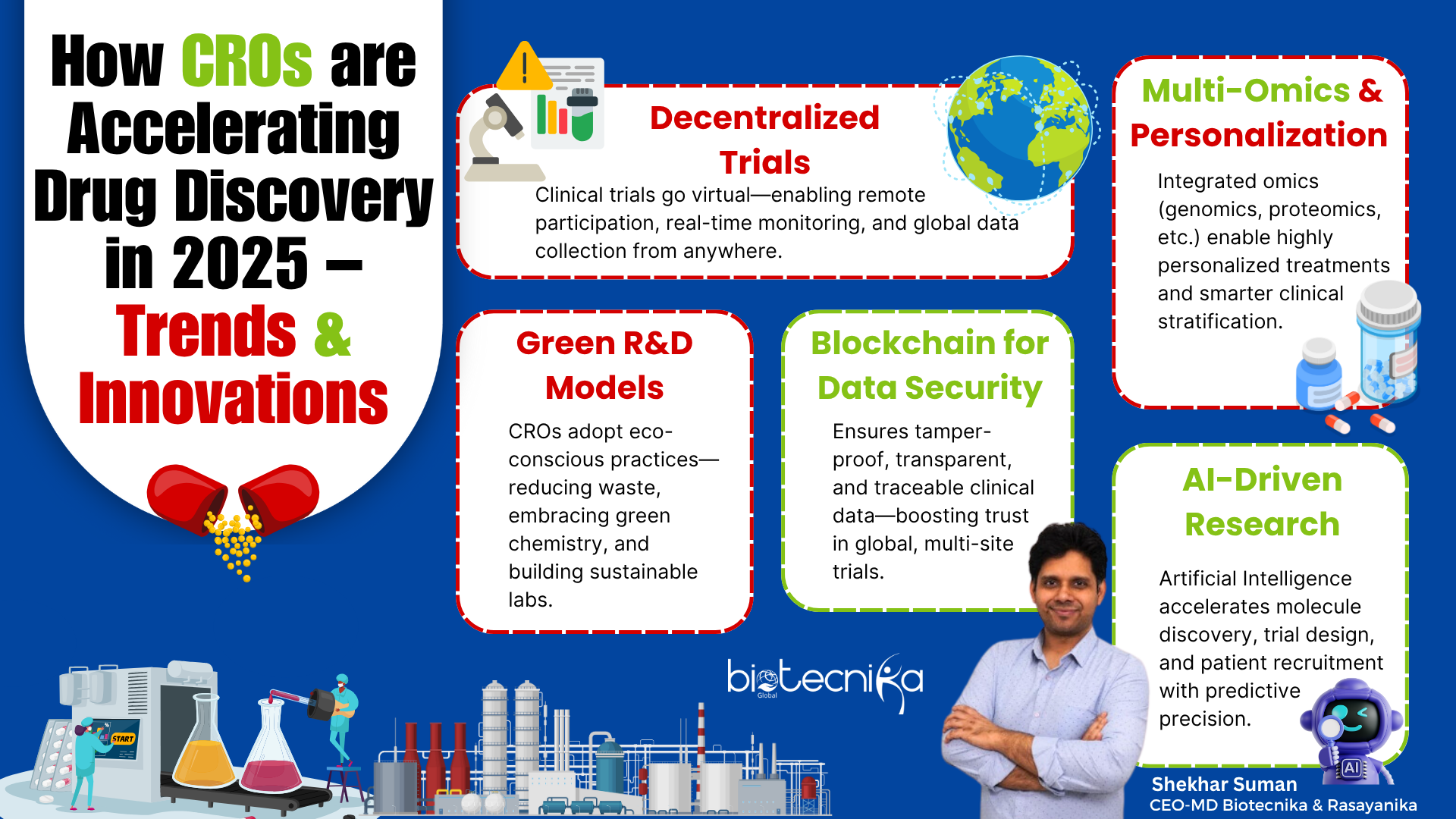
Major CRO Trends in 2025
Drug Discovery as well as R&D (Research and Development) departments are technologically evolving. This evolution is driven by shifting patient expectations, evolving regulatory environments, and the rapid advancement of technology. CROs are accelerating Drug Discovery and are at the crossroads of these global changes, and hence, they adopt innovative and novel approaches to fulfill various industries’ demands. The following Trends showcase CROs’ influence as well as expanded capabilities across the Drug Discovery ecosystem.
- Rise of Decentralized & Hybrid Clinical Trials: The Clinical Trial landscape is undergoing a revolution driven by patient-centric and Digital Technological design. DCTs (Decentralized Clinical Trials), which utilize remote monitoring tools and telemedicine, are gaining attention for their ability to engage patients and participants beyond the physical boundaries of traditional Clinical Trial sites. There are hybrid Clinical Trial models that combine virtual interactions with in-person visits, offering greater accessibility and flexibility. This aids in overcoming conventional barriers like mobility and geography. CROs are the primary implementers of these approaches, enabling more inclusive, efficient, and patient-friendly studies that generate robust data while enhancing the participant experience.
- AI-Powered Drug Discovery: Artificial Intelligence (AI) is transforming the early phases of Drug Development by enabling researchers to analyze complex and vast Biological Datasets with great accuracy as well as speed. AI algorithms aid in predicting drug-target interactions, optimizing lead compounds, as well as identifying promising molecular candidates more efficiently than conventional experimental methods. By integrating AI into their workflows, CROs reduce the expenses and time required to move Drug candidates from the discovery department to the Clinical evaluation department. This Technological leap accelerates innovation as well as opens new ways to identify complex disease treatments.
- Personalized Medicine and Integration of Multi-Omics: Personalized medicines are customized treatments to individual patients’ Biological profiles. Advancements in Multi-omics Technologies offer valuable insights into patient variability and disease mechanisms, analyzing the metabolome, proteome, genome, as well as other Biological layers. CROs utilize the Biological Data to design biomarker-driven Clinical Trials, thereby increasing the success rate of matching therapeutics to patients. This enhances resource optimization, increases Regulatory approvals, as well as better and smarter Clinical outcomes.
- Green Chemistry and Sustainable R&D: In Pharmaceutical R&D, Sustainability is becoming a significant priority, reflecting broader social and Environmental responsibilities. Green Chemistry principles (12 Fundamental Principles) focus on reducing the overall ecological footprint of Pharmaceutical facilities and laboratory processes by minimizing energy consumption, resource usage, as well as hazardous waste. CROs integrate Sustainability into their operations by adopting digital innovations and eco-friendly Technologies that promote transparency and efficiency. These Sustainable efforts align with the growing expectations of regulatory officers, patients, and sponsors who value environmental management and Sustainability as part of ethical Drug Development procedures.
- Globalization: Drug Development is becoming increasingly global, although local Drug Development expertise is essential for success in the Pharmaceutical sector. Emerging global job markets offer cost-effective infrastructure as well as diverse patient populations, making them appropriate for Clinical Trials. Navigating local cultural nuances, Healthcare systems, as well as Drug Regulations requires a specialized and skilled understanding. CROs bridge the gap by blending their reach with collaborations and a strong global presence. This accelerates clinical trial initiation, ensuring improved data representativeness as well as regulatory compliance, which are critical factors in drug approvals worldwide.
Technological CROs Innovations
The rapidly advancing Technologies are essential to CROs’ expanding capabilities. Besides utilizing only digital tools, various CROs are developing systems and proprietary platforms that transform Biological Data integrity, collaborations, and Clinical Trial design. These advanced innovations generate high-quality evidence to support Regulatory decisions, stay quite competitive in this fast-moving global market, and enhance drug as well as patient safety. CROs in the year 2025 are adopting Technology as well as pioneering futuristic tools that transform Drug Design and Discovery workflows:
- Blockchain for Trial Integrity: Blockchain Technology provides decentralized, immutable ledgers that reduce the risks of manipulation or fraud, streamlining audit processes, as well as ensuring the transparency and security of Clinical Trial Data.
- Adaptive Trial Designs: Powered by AI, adaptive protocols enable CROs to modify study parameters in real-time based on accumulating data, thereby improving efficiency and addressing ethical considerations.
- Wearable Device Integration: Continuous health monitoring via wearables generates granular, real-world patient data, enriching trial datasets and offering more profound insights into therapeutic effects.
- Cloud-Based Collaboration: Cloud platforms facilitate seamless communication among globally dispersed teams, enabling rapid data sharing, synchronized workflows, and accelerated decision-making.
Why Sponsors Are Turning to CROs More Than Ever
The escalating complexity and pace of Drug Development have led Pharmaceutical companies to seek partners who can provide more than just operational support. CROs now offer strategic value through their infrastructure, Technological assets, and Regulatory expertise. Pharmaceutical companies increasingly rely on CROs due to clear advantages:
- Speed and Scalability: CROs possess the infrastructure and expertise to launch and expand Clinical Studies across regions quickly.
- Access to Innovation: Sponsors gain immediate access to advanced Technologies, such as AI, DCT platforms, and Bioinformatics, without hefty internal investments.
- Risk Mitigation: CROs’ Regulatory knowledge helps avoid costly compliance issues and trial setbacks.
- Cost Optimization: Shared global resources and flexible contract models reduce overhead expenses.
- Focus on Core Science: Sponsors concentrate on research and commercialization while CROs manage operational complexities.
The Path Ahead & Challenges
Even though CROs are accelerating Drug discovery and are of growing prominence, the path ahead includes navigating several challenges that could impact their efficiency and effectiveness, such as:
- Trial Diversity: Ensuring demographic representation and expanding inclusivity are ongoing priorities.
- Regulatory Complexity: Navigating shifting Regulations globally requires continuous adaptation and vigilance.
- Data Standardization: Ensuring interoperability across digital platforms worldwide remains a significant challenge.
Regulatory aspects are evolving, and staying Compliant across Jurisdictions requires constant vigilance and adoption. CROs are investing in better outcome-based models, ethical AI frameworks, as well as stronger Digital ecosystems that reward results over the processes. Some emerging Technologies are:
- Quantum Computing: Promising tools to solve complex molecular structures and expedite Drug Design, albeit still in early stages.
- Public-Private Collaborations: Collaborative models to share innovation risks as well as broaden access globally.
- Digital Twins: Sophisticated Computational patient models that optimize trial protocols as well as simulate treatment responses.
- Expanded Real-World Evidence Integration: Increasing usage of real-world Biological Data to inform Regulatory decisions as well as complement Clinical Trials.
CROs have emerged as indispensable architects in this rapidly evolving Drug Discovery ecosystem, by integrating Scientific knowledge with global reach as well as advanced Technology. This era demands precision, speed, inclusivity, as well as sustainability, and CROs rise to meet these challenges, revolutionizing Drug Discovery globally. For every Biopharmaceutical company envisioning to innovate on a larger scale, a partnership with leading CROs has become an important success.
They combine Technological advancements with Scientific expertise, and are transforming how therapeutics are tested, developed, discovered, as well as launched in the global market.
For established or emerging Pharmaceutical giants and Biotechnology startups, collaborating with a forward-thinking CRO is becoming critical. Together, they drive breakthroughs that promise Personalized treatments, Sustainable practices, as well as faster cures, thereby transforming global health one molecule, one patient, and one trial at a time.