Quality Assurance Jobs in Germany
This exciting Quality Assurance Jobs opportunity in Germany is ideal for professionals seeking to advance their careers in regulated life science environments. Based in Dreieich near Frankfurt, this role at Bio-Rad Laboratories offers a strong job opportunity for life science graduates and experienced QA professionals who want to work with global quality systems, GMP compliance, and regulatory excellence in the healthcare industry.
About Bio-Rad Laboratories
Bio-Rad Laboratories is a global leader in life science research and clinical diagnostics, with over 7,700 employees worldwide. For more than 70 years, Bio-Rad has been advancing science and healthcare by developing innovative products that support molecular biology, biochemical research, and clinical diagnostics—helping people around the world live longer and healthier lives.
Job Details:
- Job Title: Quality Assurance Specialist (m/f/d)
- Job ID: 2025-37606
- Location: Dreieich, Hesse, Germany
- Department: Quality & Regulatory
- Work Model: Hybrid
About the Role
The company is seeking a Quality Assurance (QA) Specialist (m/f/d) to support products and processes at our production site in Dreieich near Frankfurt am Main. In this role, you will serve as the primary point of contact for quality-related matters on site, collaborate closely with experienced QA specialists and cross-functional departments, and ensure compliance of the quality management system with regulatory requirements. You will actively monitor operational processes and drive continuous improvement initiatives.
What You Can Achieve?
- Act as a QA expert for product-related questions and issues, both internally and in communication with regulatory authorities
- Serve as the first deputy to the Validation Specialist and provide needs-based support for validation activities
- Manage CAPA, Change, and Non-Conformance (NC) cases, including formal and product-related review and approval
- Support the revision of relevant SOPs related to CAPA, Changes, and Quality Notifications (QNs)
- Conduct training sessions on CAPA, Changes, and QNs as required
- Perform internal audits and actively support government inspections and external audits
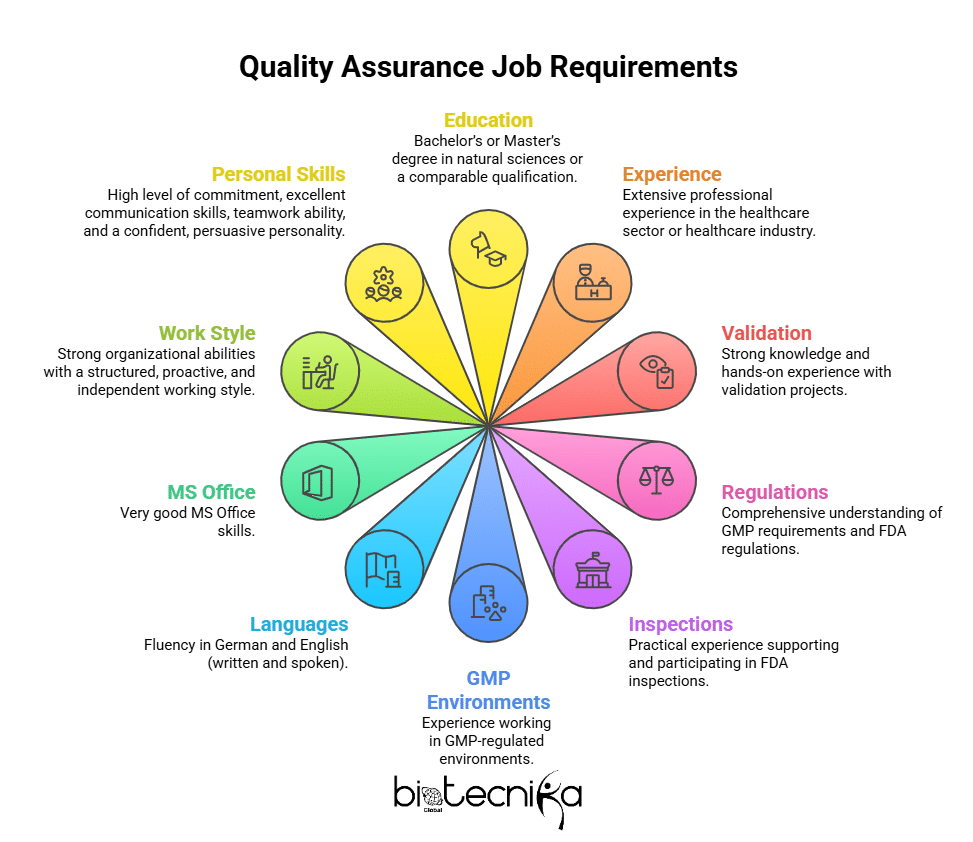
What You Bring?
- Bachelor’s or Master’s degree in natural sciences or a comparable qualification
- Extensive professional experience in the healthcare sector or healthcare industry
- Strong knowledge and hands-on experience with validation projects
- Comprehensive understanding of GMP requirements and FDA regulations
- Practical experience supporting and participating in FDA inspections
- Experience working in GMP-regulated environments
- Fluency in German and English (written and spoken)
- Very good MS Office skills
- Strong organizational abilities with a structured, proactive, and independent working style
- High level of commitment, excellent communication skills, teamwork ability, and a confident, persuasive personality
What Bio-Rad Laboratories Offers?
- Competitive annual salary based on the chemical industry collective agreement
- Comprehensive benefits and social security package
- Annual performance reviews linked to contribution and development
- 30 days of paid vacation per calendar year plus vacation pay
- Family-friendly flexible working hours
- Hybrid working model with the option to work remotely up to two days per week