Regulatory Compliance
Imagine that you have created a new energy drink. You prepared it in your home kitchen by mixing some ingredients. It tastes great and can help you stay awake for your final exams. You thought of selling it to everyone in your school. But how do people know that it is safe to drink? What if people who consume it may fall sick? What if it contains too much caffeine? Before you sell a single bottle, you need to prove that it is safe. You should follow the rules and regulations set by the school, the city, and even the government. This process is called Regulatory Compliance in the Life Science Industry.
Regulatory Compliance is the “rulebook” that keeps us safe from bad products and dangerous devices. This article will introduce you to the world of regulatory affairs, quality assurance, and the global rules that keep the world healthy.
What is meant by Regulatory Compliance?
Regulatory Compliance means following the laws. Compliance is not just about avoiding fines in the Life Science and Biotech Industries that make drugs, vaccines, and medical devices. It is more about saving lives.
If you take a pill for a headache or get a flu injection, you always trust that it will not hurt you in any of the ways. This trust is all due to a strict compliance framework. A compliance framework is like the blueprint of a house. It ensures a company acts, tests, and reports its data to ensure everything is in order.
Companies may not operate effectively without a strong compliance framework. They might do everything by guess instead of obeying the norms. That is why there are global watchdogs.
The Big Watchdogs
The Life Sciences industry has regulatory agencies, just as a game has referees. The regulatory agencies around the world are listed below,
-
FDA Regulations (The American Guardian)
- The FDA stands for the Food and Drug Administration. They are the strict principal of the United States. FDA Regulations are famous for being very detailed and tough. If a company wants to sell a medicine in the US, it must comply with FDA regulations in full.
- FDA Regulations cover everything, such as how to test a drug on animals, how to test it on humans (clinical trials), and what the label on the bottle must say. No matter how good your product is, you cannot sell it in America if you don’t follow FDA Regulations.

-
EMA Compliance (The European Team)
- EMA stands for the European Medicines Agency. EMA compliance is similar to FDA compliance but covers many countries across the European Union.
- Achieving EMA compliance requires significant teamwork. Europe has many countries with different languages. EMA compliance ensures that a patient in Germany and a patient in France both receive the same safer medicine, with instructions they can understand.
-
ISO Standards
- The International Organization for Standardization is essential for medical devices, diagnostics, and quality systems.
- ISO doesn’t approve products. It ensures processes stay clean, safe, and consistent.
-
CDSCO (Indian Landscape)
- The Central Drugs Standard Control Organisation is the regulatory landscape in India.
- With India emerging as a top vaccine and biologics manufacturer, CDSCO has expanded its guidelines, digital processing, and quality expectations.
- The system is aligning more closely with global benchmarks, making Indian biotech increasingly competitive.
-
WHO Guidelines (The Global Guide)
- The World Health Organization is like the world’s wise grandparent. They don’t make laws for just one country. They create WHO guidelines for the whole planet.
- WHO guidelines are especially important for developing countries that might not have their own strict agencies. When a global health crisis occurs (such as a pandemic), everyone looks to the WHO guidelines to determine how to make vaccines safe for billions of people.

What Happens When the Rules Break?
You may wonder why there is so much paperwork in regulatory processes. The answer is simple. The risks are too high to ignore. If a company ignores the compliance framework, bad things can happen.
-
Safety of the Patient
This is the major risk. A medicine could be toxic if quality assurance fails. It may contain a harmful microorganism, or it might not work. This can lead to unexplained sickness or even death.
-
Legal Issues and Fines
When the companies break the rules, the regulatory authorities do not like it. Ignoring the rules can lead to massive fines, sometimes billions of dollars. The company owners can even be imprisoned.
-
Damage to the Reputation
A company’s reputation is destroyed if it fails to follow regulatory requirements and harms patients. People do not prefer buying products from them again.
-
Business Shutdown
If a company refuses to follow the WHO guidelines or local laws, the regulatory authorities would shut down the business completely. This means that people lose their jobs, and patients lose their access to medicines they need.
The High Cost of Non-Compliance
| Risk Category | What Happens? | Real-World Example |
| Patient Health | Sickness, injury, or loss of life. | A company sells a cough syrup with toxic ingredients. |
| Financial | Huge fines and loss of sales. | A company pays $1 billion for hiding safety data. |
| Legal | Lawsuits and prison time for bosses. | Executives arrested for faking test results. |
| Operational | Factories are closed by the FDA/EMA. | Production stops, causing drug shortages. |
Quality Assurance and Regulatory Affairs
Pharmaceutical and Biotech companies hire specialized teams to prevent unwanted issues.
Quality Assurance and Regulatory Affairs are the heroes behind the scenes.
-
Quality Assurance (The Inspectors)
Quality Assurance is like the final boss in a video game that you have to beat to move to the next level. Everything is checked by the QA team. They check the raw materials, the machines, the floor cleanliness, and the final product.
Everything will be stopped when a Quality Assurance Officer finds a mistake. They act as a shield for consumers.
-
Regulatory Affairs (The Diplomats)
Regulatory Affairs is the bridge between the company and the government. Whereas QA operates within the factory.
The Regulatory Affairs team reviews the rulebooks to ensure the company complies. They study FDA Regulations, master EMA Compliance, and interpret WHO guidelines. They are the ones who fill thousands of pages of documents required for getting a product approved.
The regulatory affairs expert figures out how to get a medicine to the people in a legal way when a scientist investes a new cure. They ensure that the company stays within the compliance framework. This prevents the company from getting into trouble.
Building a Strong Compliance Framework
So, how does a company actually follow all these rules? They build a compliance framework. This compliance framework is a system of policies and habits.
-
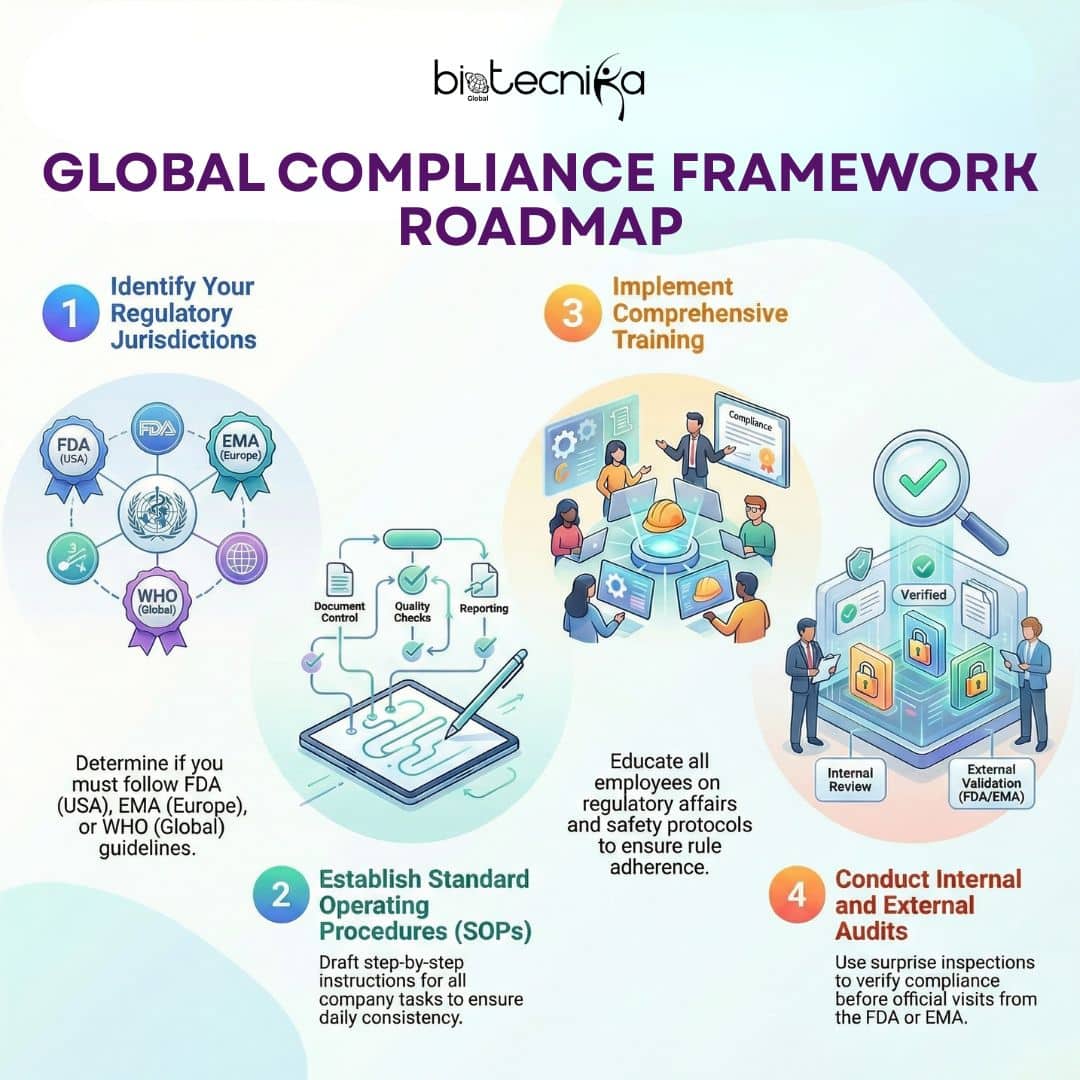
Step 1: Know the Rules
First, you must know which rules apply to your company and the product. If you want to sell your product in the USA, you should know about FDA Regulations. If you are selling it in Europe, you must focus on EMA Compliance. If you are selling it around the world, look for WHO guidelines.
-
Step 2: Write it Down (SOPs)
Companies write “Standard Operating Procedures” (SOPs). These are step-by-step instructions for everything.
- How to wash hands?
- How to turn on the mixer?
- How to write a report?
- The Quality assurance teams ensure these SOPs are followed every single day.
-
Step 3: Training
You can’t follow rules if you don’t know them. The companies spend a lot of time to teach their employees about regulatory affairs and safety.
-
Step 4: Audits (The Pop Quiz)
An audit is like a surprise inspection. The Quality Assurance teams randomly check the departments in a company to make sure that they follow the compliance framework. Sometimes, the FDA or EMA will visit for an external audit. If you pass, great! If not, you have a lot of work to do.
Global Standards: Connecting the World
The world is a big place. Sometimes, the FDA Regulations differ from EMA compliance. This can be confusing and hard to understand. That is why there is a push for “Harmonization.” This harmonization means to get different countries agree on similar standards.
The WHO guidelines help with this harmonization. They aim to establish a baseline everyone can agree on. This is also called the ICH (International Council for Harmonization). This is a group where regulatory affairs experts from different countries sit together and agree on principles of scientific standards. This helps companies build a single compliance framework that works across multiple countries simultaneously.
Key Differences Between Agencies
| Feature | FDA Regulations (USA) | EMA Compliance (Europe) | WHO Guidelines (Global) |
| Jurisdiction | United States only. | European Union (multiple countries). | Worldwide (Advisory). |
| Power | Can ban products and directly fine companies. | Coordinates approvals for member countries. | Sets standards but cannot force countries to follow them. |
| Focus | Very strict on safety data and factory inspections. | Focuses on harmonizing rules across different cultures. | Focuses on access to medicines for everyone, everywhere. |
The Future of Regulatory Compliance
The world of Quality Assurance and Regulatory Affairs is changing fast.
- Technology and AI: Artificial Intelligence can be used to identify safety issues. Computers can scan thousands of documents to ensure the compliance framework is strong. However, even AI needs to follow FDA Regulations to ensure compliance.
- Speed vs. Safety: Vaccines were developed more quickly during the COVID-19 Pandemic. This was a triumph of regulatory affairs. The agencies identified ways to complete the discovery process without violating the WHO safety standards. We could hope for more rapid approvals of life-saving drugs in the future.
- Patient Focus: Modern Quality Assurance is more about the consumer than the product. Companies are now listening more to consumers and designing compliance frameworks based on their needs. The compliance framework is becoming more “consumer-centric.”
Conclusion
Regulatory compliance might sound boring at first. It’s a lot of paperwork, rules, regulations, and surprise inspections. But remember the energy drink story? We wouldn’t know what we are putting into our bodies without these rules.
FDA Regulations protect us in America. EMA Compliance keeps Europe safe. WHO guidelines prioritize global safety. The hardworking Quality Assurance and Regulatory Affairs Professionals act as the guardians of health in each and every biotech and pharma company. They ensure that when a doctor prescribes a medicine, it helps you rather than harming you. They build the compliance framework that holds the entire life sciences industry together. So, the next time you take a vitamin tablet or get a vaccine, remember the hidden heroes behind the compliance who ensured it was safe for consumption.



