Start Your Career with WuXi Biologics!
Looking to start your career in Quality Assurance within the biologics industry? The Quality Assurance Intern position at WuXi Biologics in Cranbury, NJ, offers an exciting Summer 2026 internship opportunity. This Quality Assurance Internship in the USA provides hands-on experience in GMP document management, regulatory compliance, and QA systems within a globally recognized biologics development and manufacturing company.
Internship Details:
- Title: Quality Assurance Intern
- Requisition ID: 5322
- Location: Cranbury, New Jersey
- Country: United States
- Job Type: Summer Internship (2026)
- Department: Quality Assurance
- Pay Range: $20–30 per hour
About the Company:
WuXi Biologics is a global leader in biologics development and manufacturing, supporting more than 600 customers worldwide, including all top 20 biopharma companies. With over 12,000 team members, the company drives innovation through its PROUD culture—Passion, Reward, Opportunity, Unity, and Determination. WuXi Biologics plays a critical role in shaping the future of biologics and advancing healthcare innovation globally.
Educational Requirements for the Internship:
- Bachelor of Science or higher degree in Microbiology, Biochemistry, or equivalent field
- Proficiency in computer software
- Strong communication, teamwork, and organizational skills
Key Responsibilities:
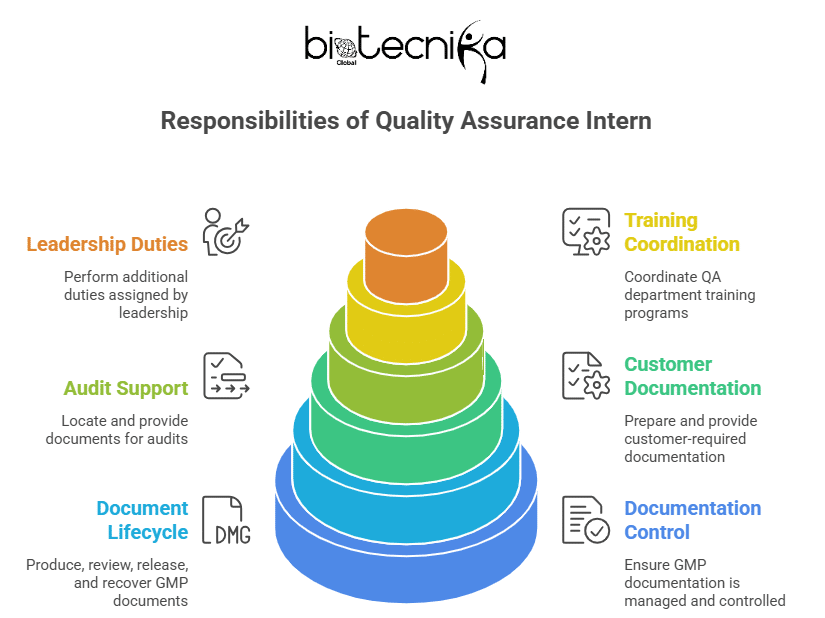
- Manage and control GMP documentation in the Quality Assurance Internship in the USA
- Produce, review, release, and recover GMP documents and blank record forms
- Supervise the implementation of quality document management processes across departments
- Maintain and manage electronic document systems and support new system launches
- Maintain and train staff on document processes in the MC system
- As a Quality Assurance Intern, you should distribute, recycle, scan, and archive BPR and analysis records
- Manage archiving, loaning, and destruction of executed records
- Label and shelve archived documents
- Scan archived records and maintain electronic copies
- Prepare and provide customer-required documentation
- Support audits by locating and providing the required documents in the Quality Assurance Internship
- Coordinate QA department training
- Perform additional duties assigned by leadership
Skills Required:
- Strong understanding of GMP documentation and regulatory compliance
- Excellent communication and teamwork skills
- Strong organizational and record-keeping abilities
- Proficiency in computer systems and document management tools
- Attention to detail and the ability to manage multiple tasks
Benefits of the Quality Assurance Internship:
- Competitive internship pay ($20–30/hr)
- Hands-on experience in GMP and regulatory compliance
- Exposure to global biologics manufacturing operations
- Opportunity to work with industry leaders
- Career-building experience in a top-tier biopharma organization