Clinical Trial Associate Role at Menarini Stemline USA
Explore a dynamic clinical trial job with Menarini Stemline in New York. As a Clinical Trial Associate, you’ll support oncology studies, manage trial documentation, and ensure regulatory compliance while contributing to life-changing therapies.
Job Details:
- Location: New York, United States
- Job Type: Full-time, Corporate Office
- Salary: $115,000–$155,000 per year
- Experience Required: Minimum 2 years in clinical research; oncology experience required
- Reporting To: Clinical Program/Trial Lead
- Travel: Minimal, mostly office-based
About Menarini Stemline:
Menarini Stemline, part of the Menarini Group founded in 1886, is a global biopharmaceutical leader focused on oncology. With operations in 70 countries and cutting-edge therapeutics and diagnostics, the company pioneers therapies like Tagraxofusp and Selinexor, and advanced liquid biopsy diagnostics via Menarini Silicon Biosystems. Explore more clinical trial job offers.
Educational Requirements:
B.A./B.S. in Life Sciences or equivalent experience
Key Responsibilities for Clinical Trial Associate Role:
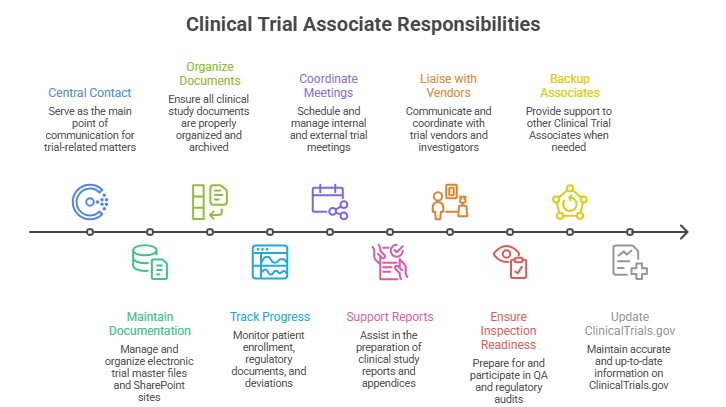
- Act as central contact for trial communications and documentation
- Maintain eTMF and trial SharePoint site, prepare for audits
- Organize and archive clinical study documents
- Track patient enrollment, regulatory documents, AE/SAE, and protocol deviations
- Coordinate internal/external meetings, agendas, and minutes
- Support Clinical Study Reports and appendices
- Liaise with trial vendors, investigators, and third-party payments
- Ensure inspection readiness and participate in QA/regulatory audits
- Act as backup for other Clinical Trial Associates as needed
- Update clinical trial status on ClinicalTrials.gov
Skills Required for Clinical Trial Associate Role:
- Knowledge of ICH-GCP, FDA regulations, and clinical trial best practices
- Proficiency with eTMF platforms, Microsoft Suite, Adobe Acrobat, and Smartsheet
- Strong organizational, written, and verbal communication skills
- Attention to detail, ability to manage multiple projects under tight deadlines
- Ability to handle sensitive information and collaborate with cross-functional teams
Benefits of the Clinical Trial Associate Role:
- Competitive base salary: $115,000–$155,000
- Short-Term Incentive Programs
- Fidelity 401(k) with company match
- Anthem Premier PPO and HDHP insurance plans
- Company-paid Basic Life & AD&D insurance, pre-tax FSA/HSA programs
- Opportunity to contribute to innovative oncology research