Clinical Trial Jobs at Medpace Singapore
Looking to advance your career through high-impact clinical trial jobs? This Clinical Trial Manager opportunity in Singapore offers experienced professionals and ambitious candidates a unique job opportunity for life science graduates to lead global infectious disease and immunology studies. Among the most competitive Jobs in Singapore, this role at Medpace combines leadership, scientific rigor, and international exposure.
About Medpace
Medpace is a global, full-service clinical contract research organization supporting Phase I–IV trials across major therapeutic areas. With over 5,000 employees in 40+ countries, jobs at Medpace provide access to world-class research infrastructure and some of the most respected clinical trial jobs globally, including premier Jobs in Singapore.
Job Details:
- Job Title: Clinical Trial Manager – Infectious Disease / Immunology
- Location: Singapore, Singapore
- Function: Clinical Trial Management
- Job ID: 12033
Clinical Trial Job Summary
Medpace is seeking an experienced Clinical Trial Manager (CTM) to lead global clinical research studies. Medpace is a scientifically driven, full-service contract research organization (CRO) that empowers project teams to independently lead and execute clinical trials.
Clinical Trial Managers with expertise in Immunology and Infectious Diseases are encouraged to continue developing within their therapeutic area. The CTM serves as a key liaison between sponsors and investigational sites, managing study timelines and project deliverables (excluding financial oversight).
This role coordinates all contracted services for a study and leads cross-functional teams across the full project lifecycle—from study start-up through database lock and study closure. The CTM may also participate in bid defense meetings and support business development activities as needed.
This is a fully office-based position located in Medpace’s Singapore office.
Responsibilities
- Manage and maintain accountability for day-to-day project operations in accordance with contract requirements, ICH/GCP guidelines, and applicable laws and regulations
- Serve as the primary sponsor contact for project-specific issues and study deliverables
- Maintain in-depth knowledge of the study protocol, therapeutic area, and indication
- Provide cross-functional oversight of project team members and ensure completion of all deliverables
- Ensure all required project-specific training is completed by study team members
- Review and contribute to study documents, including protocols, edit check specifications, data analysis plans, and final study reports, as applicable
- Develop and maintain operational project plans
- Conduct risk assessments and implement mitigation strategies
- Manage study vendors
- Oversee site quality, including direct supervision of Clinical Research Associates (CRAs) and monitoring deliverables
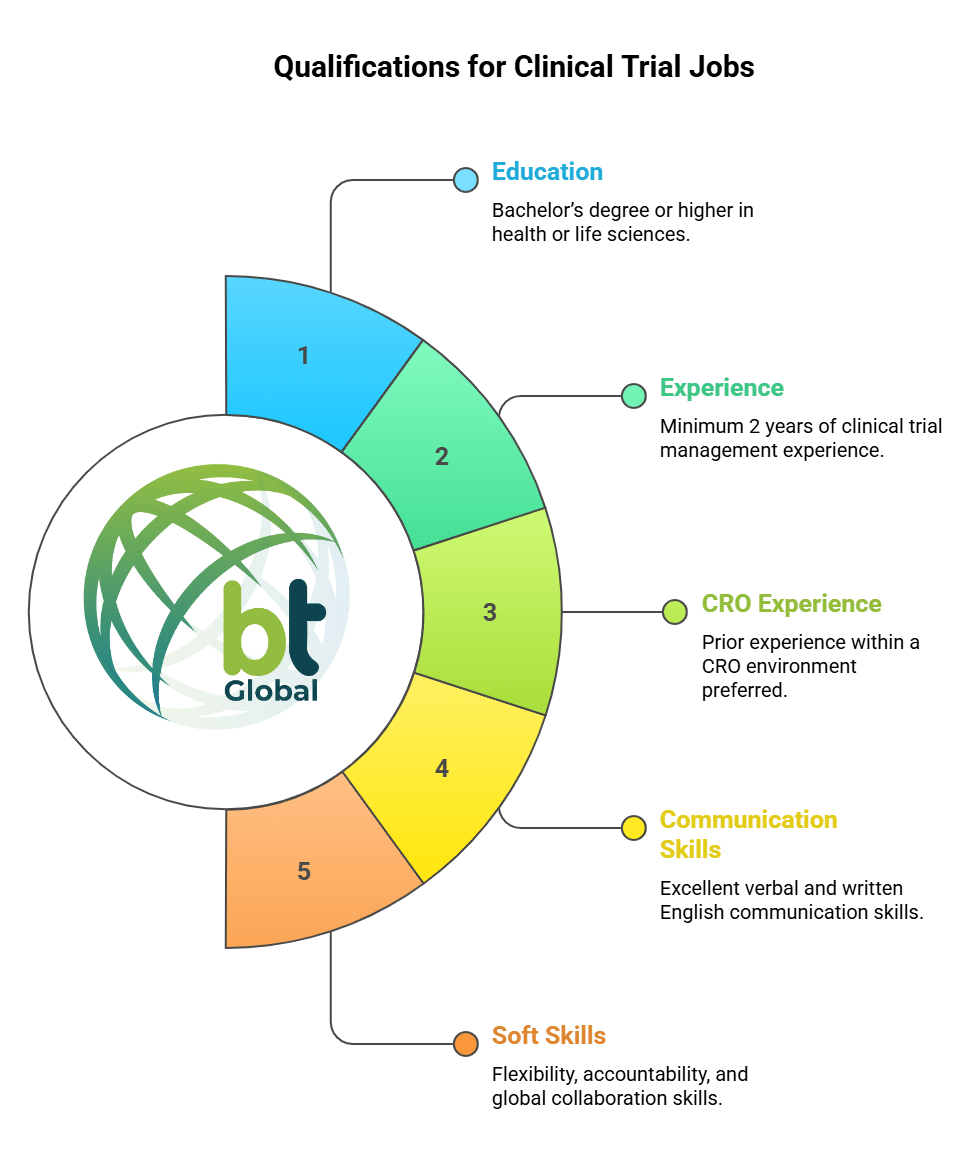
Qualifications for Clinical Trial Jobs
- Bachelor’s degree or higher in a health or life sciences–related field
- Minimum of 2 years of clinical trial management experience in the Asia-Pacific region (experience in relevant indications is a plus)
- Prior experience within a CRO environment preferred
- Excellent verbal and written English communication skills
- Ability to work flexibly, take accountability, and operate effectively in a global environment
Why Medpace?
For over 30 years, Medpace’s work has positively impacted the lives of patients and families affected by hundreds of diseases across all major therapeutic areas. The work conducted today continues to shape better outcomes for patients in the future.
Perks of a Clinical Trial Job
- Flexible work environment
- Competitive compensation and benefits package
- Competitive paid time off (PTO)
- Structured career paths with opportunities for professional growth
- Company-sponsored employee appreciation events
- Employee health and wellness initiatives