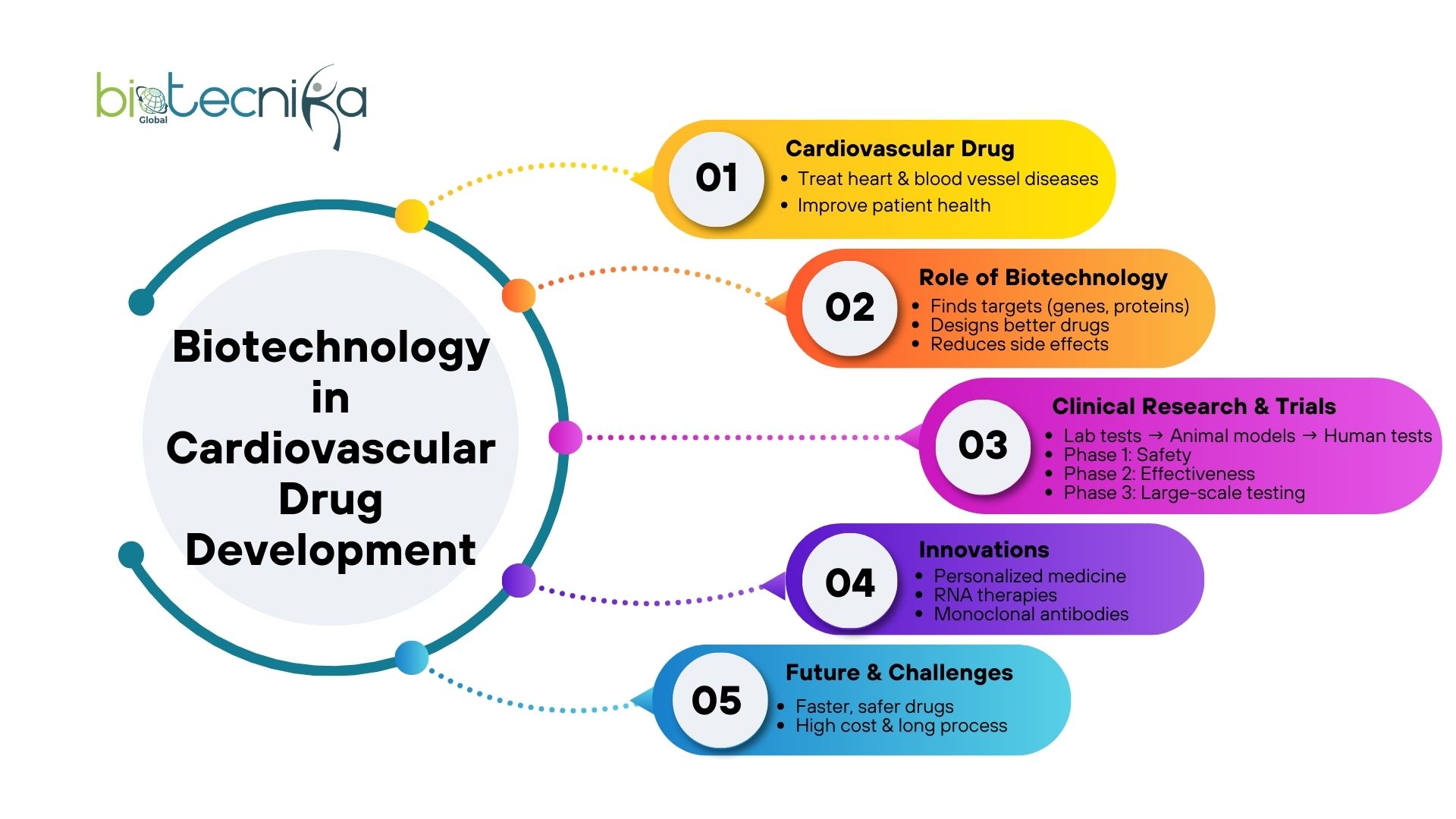
The Role of Biotechnology in Cardiovascular Drug Development in the United States
Cardiovascular disease is one of the main diseases that has the potential to cause death in the United States. Every year, millions of people are affected by heart issues. So, it is very important to develop drugs that could effectively treat cardiovascular diseases. For the past few years, the Role of Biotechnology has helped a lot in making new cardiovascular Drug and treatments. This makes a difference in helping patients get better. Starting from identifying molecular targets to testing in human subjects. Biotechnology is helping to change how cardiovascular medicines are developed in clinical research.
Understanding Cardiovascular Drug Development
Before any new heart medicine is sold in stores, it undergoes several steps and tests. Firstly, the scientists will identify the molecular targets in the heart or blood vessels. Then they will find a part or portion that may help them to treat or prevent heart disease. Some of the biotechnology tools, like gene editing, protein engineering, and cellular assays, will help researchers design drugs. This will help the researchers to target these molecules correctly. This accuracy reduces the side effects after treatment. Also, the effectiveness of the treatment will increase.
How Biotechnology Helps in Clinical Research
The role of biotechnology is very important in clinical research. Once a potential cardiovascular drug is discovered, it must go through tough testing in order to ensure safety and effectiveness. Biotechnology helps this process by providing advanced models. These models include genetically engineered cells and animals. These cells and animals can mimic human cardiovascular conditions. These models allow scientists to predict how a drug might behave in humans. This process of mimicking helps the researchers to speed up the transition from lab research to clinical trials.
In the United States, clinical trials are strictly regulated to protect patients and ensure reliable results. Biotechnology helps scientists closely study how the body reacts at a very small level. This makes it easier to find the best cardiovascular drugs. This approach reduces the risks. This helps in bringing effective drugs to the market faster.
From Laboratory to Clinical Trials
Once a drug has successfully passed the lab testing, the drug enters the first phase of clinical trials. The first phase of clinical trials usually involves a small group of volunteers. Here, scientists study the safety and dosage of the new drug. The role of biotechnology is still very important in providing tools to measure the biomarkers. Then the drug will be monitored to see how it interacts with the body.
Once the first phase of clinical trials is completed successfully, then larger trials are conducted in order to test the drug’s effectiveness in treating cardiovascular diseases. During this stage, the clinical research relies heavily on biotechnology to analyse complex data. Here, biotechnology is used to identify side effects. This helps in understanding how the drug will impact the long-term. The final phase of clinical trials makes sure that the medicine is both safe and effective. This is ensured before the drug gets approved by the Food and Drug Administration (FDA).
Innovations Shaping Cardiovascular Drug Development
There are multiple advancements in drug development through biotechnological means. Monoclonal antibodies, RNA-based therapies, and personalized medicine are becoming increasingly prevalent as forms of biotechnological innovation in cardiovascular drug therapies. By using these advanced techniques to create new drugs that target a patient’s specific genetic and/or molecular profile for their cardiovascular condition, researchers are now able to improve patient outcomes.
Additionally, through the combination of biotechnological research, digital tools, and artificial intelligence, biotechnologists can utilize large amounts of data collected from clinical trials to make better decisions and create more effective cardiovascular medications that meet the needs of individual patients.
Challenges in Cardiovascular Drug Development
While developing a cardiovascular medication has become more efficient than in the past, there are still challenges with high costs, long-duration clinical trials, and regulatory hurdles that slow the drug development process. The field of biotechnology is also developing new technologies that will allow for the development of new classes of cardiovascular drugs. For example, advanced laboratory techniques, predictive modelling systems, and enhanced databases regarding human biology are aiding in the efficient development of cardiovascular drugs.
The Future of Cardiovascular Drug Research
The forecast for the future of biotechnology is optimistic through continued innovation within the areas of biotech. It is expected that as Biotech advances, there will be increased development of new cardiovascular medications which will be more efficacious and safer than those presently available. Additionally, through the continued use of genetic testing, personalised medicine, and advanced research methodologies, it is expected that the biomedicine sector will evolve significantly. Ultimately, this will produce improved results for patients and decreased prevalence of cardiovascular disease in the US.
Conclusion
The role of Biotechnology is an essential component of cardiovascular drug development in the United States. The use of biotechnology relates to everything from discovering molecular targets to developing advanced clinical trial methods. Biotechnology’s goal is to provide new drugs that are safe, effective, and tailored to the patient’s unique needs through continued innovation and research. The future of cardiovascular medicine is extremely promising.