Clinical Research Job at ICON USA
Are you looking for a rewarding clinical research job that offers national reach, professional growth, and real impact on global clinical trials? This Clinical Site Associate role represents an excellent jobs opportunity for life science graduates and early-career professionals seeking jobs in USA within a world-leading clinical research organization. ICON’s inclusive culture and global footprint make this clinical research job a standout opportunity in today’s competitive market.
About ICON plc
ICON plc is a global leader in clinical research and healthcare intelligence, partnering with pharmaceutical, biotechnology, and medical device organizations worldwide. Known for offering high-quality clinical research jobs, ICON provides exceptional job opportunities for life science graduates and experienced professionals across the USA and beyond. The company’s people-first culture, innovation focus, and global scale make it a top employer in clinical development.
Job Details:
- Job Title: Clinical Site Associate (CSA)
- Job ID: JR139929
- Location: Multiple U.S. Locations (Remote/Home-Based Eligible)
- Function: Clinical Monitoring – ICON Full Service & Corporate Support
- Experience Level: Minimum 1 year in clinical research
About the Clinical Research Job Role
ICON is seeking a Clinical Site Associate (CSA) to support clinical trial operations across the United States. This role is open to candidates located anywhere in the U.S. As a CSA, you will play a key role in ensuring site compliance, documentation accuracy, and audit readiness, working closely with Clinical Research Associates (CRAs) to support the successful delivery of clinical trials.
Key Responsibilities
Site Support & Oversight
- Manage site-level communications
- Coordinate site trainings and system access
- Support site readiness activities, including pre- and post-site visit tasks
- Follow up on action items and outstanding issues
Document Management
- Maintain the Trial Master File (TMF)
- Ensure accuracy, completeness, and inspection readiness of documentation
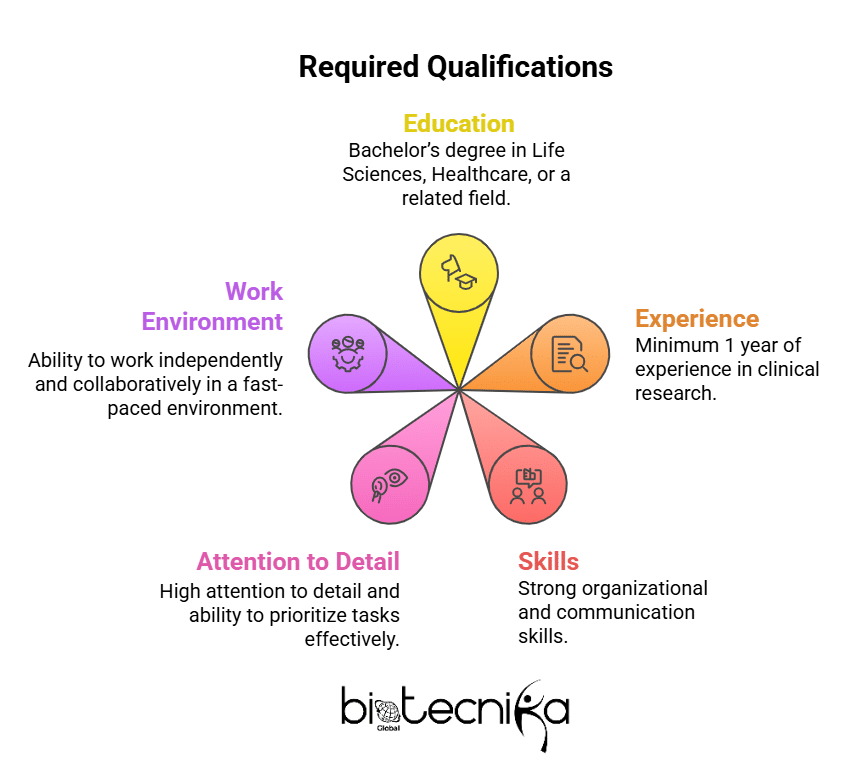
Required Qualifications
- Bachelor’s degree in Life Sciences, Healthcare, or a related field
- Minimum 1 year of experience in clinical research
- Strong organizational and communication skills
- High attention to detail and ability to prioritize tasks effectively
- Ability to work independently and collaboratively in a fast-paced environment
Benefits May Included in Clinical Research Job:
- Competitive salary
- Generous annual leave entitlements
- Comprehensive health insurance options for you and your family
- Competitive retirement planning programs
- Global Employee Assistance Programme (LifeWorks) with 24/7 access to over 80,000 specialists
- Life assurance
- Flexible, country-specific benefits such as:
- Childcare vouchers
- Bike purchase schemes
- Discounted gym memberships
- Subsidized travel passes
- Health assessments
Why Join ICON?
By joining ICON, you’ll be part of a global organization where your work directly contributes to advancing clinical research and improving patient outcomes—while enjoying strong support for work-life balance and professional development.